Bromothymol blue and carbon dioxide
Bromothymol Blue And Carbon Dioxide. The btb will turn yellow after you ve blown into it this change occurs because the carbon dioxide in your breath is acidic and btb is an indicator. When carbon dioxide is added to the solution it creates carbonic acid lowering the ph of the solution. It is mostly used in applications that require measuring substances that would have a relatively neutral ph. When it turns yellow it is overshot and.
 Bromothymol Blue Changing Colors With Only A Breath Youtube From youtube.com
Bromothymol Blue Changing Colors With Only A Breath Youtube From youtube.com
When it turns green it is at the endpoint of the titration. As the carbon dioxide is absorbed from the breath into the solution the solution changes its color to yellow from green by forming carbonic acid. Bromthymol blue changes color over a ph range from 6 0 yellow to 7 6 blue. Co2 in water makes a slightly acidic carbonic acid. Place bromothymol blue in a flask and use a straw to blow into the liquid. A common use is for measuring the presence of carbonic acid in a liquid.
We use bromothymal blue as an indicator to determine if the solution is acidic.
When carbon dioxide is added to the solution it creates carbonic acid lowering the ph of the solution. We use bromothymal blue as an indicator to determine if the solution is acidic. Bromothymol blue bmb is an indicator dye that turns yellow in the presence of acid. Bromthymol blue indicates carbon dioxide is present. When carbon dioxide is added to the solution it creates carbonic acid lowering the ph of the solution. As the carbon dioxide is absorbed from the breath into the solution the solution changes its color to yellow from green by forming carbonic acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A common demonstration of the ph indicator properties of btb involves exhaling through a tube into the neutral solution of bromothymol blue. This change was indicated by bromothymol blue seeing that the substance reverted from a green color to its yellow acidic state. As the carbon dioxide is absorbed from the breath into the solution the solution changes its color to yellow from green by forming carbonic acid. Bromthymol blue changes color over a ph range from 6 0 yellow to 7 6 blue. Color may vary when you first open the container.
 Source: slideplayer.com
Source: slideplayer.com
When it turns green it is at the endpoint of the titration. Co2 h2o h2co3 h hco3 the h protonate the bromothymol blue which becomes yellow. When carbon dioxide is added to the solution it creates carbonic acid lowering the ph of the solution. It is mostly used in applications that require measuring substances that would have a relatively neutral ph. The exhalation of carbon dioxide into the substance created a carbonic acid which led to a change in the ph level.
 Source: youtube.com
Source: youtube.com
Co2 h2o h2co3 h hco3 the h protonate the bromothymol blue which becomes yellow. The btb will turn yellow after you ve blown into it this change occurs because the carbon dioxide in your breath is acidic and btb is an indicator. Co2 in water makes a slightly acidic carbonic acid. It is mostly used in applications that require measuring substances that would have a relatively neutral ph. It is also a general acid base indicator.
 Source: youtube.com
Source: youtube.com
It is also a general acid base indicator. Bromthymol blue indicates carbon dioxide is present. It is typically sold in solid form as the sodium salt of the acid indicator. The exhalation of carbon dioxide into the substance created a carbonic acid which led to a change in the ph level. Place bromothymol blue in a flask and use a straw to blow into the liquid.
 Source: sciencelessonsthatrock.com
Source: sciencelessonsthatrock.com
This change was indicated by bromothymol blue seeing that the substance reverted from a green color to its yellow acidic state. It is mostly used in applications that require measuring substances that would have a relatively neutral ph. Place bromothymol blue in a flask and use a straw to blow into the liquid. This change was indicated by bromothymol blue seeing that the substance reverted from a green color to its yellow acidic state. It is also a general acid base indicator.
 Source: howtosmile.org
Source: howtosmile.org
When it turns green it is at the endpoint of the titration. For drama make sure you put on safety goggles. We use bromothymal blue as an indicator to determine if the solution is acidic. A common use is for measuring the presence of carbonic acid in a liquid. When it turns green it is at the endpoint of the titration.
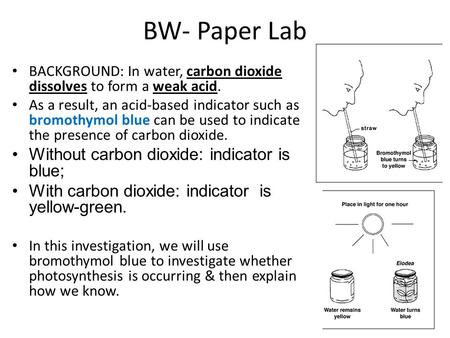 Source: slideplayer.com
Source: slideplayer.com
The exhalation of carbon dioxide into the substance created a carbonic acid which led to a change in the ph level. Bromthymol blue indicates carbon dioxide is present. It is also a general acid base indicator. It will change colors i. It is mostly used in applications that require measuring substances that would have a relatively neutral ph.
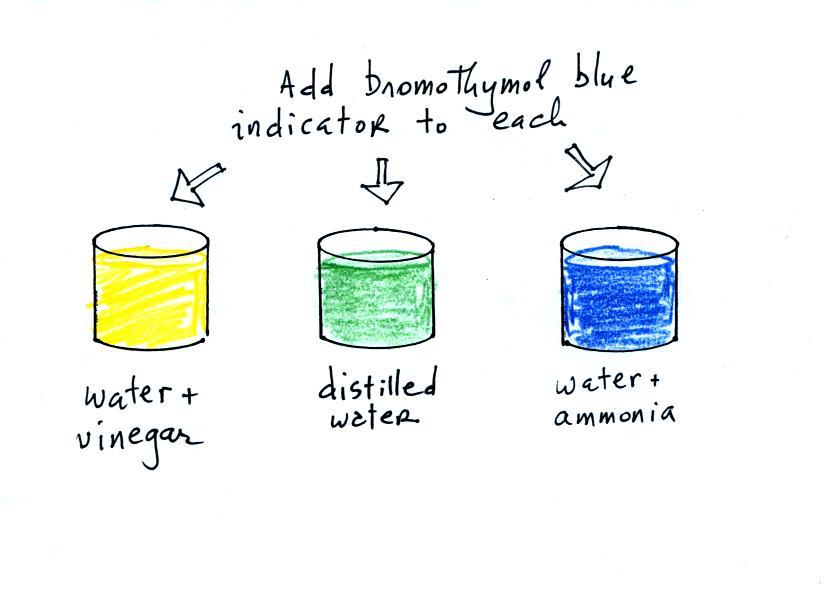 Source: atmo.arizona.edu
Source: atmo.arizona.edu
Bromthymol blue changes color over a ph range from 6 0 yellow to 7 6 blue. Place bromothymol blue in a flask and use a straw to blow into the liquid. This change was indicated by bromothymol blue seeing that the substance reverted from a green color to its yellow acidic state. A common demonstration of the ph indicator properties of btb involves exhaling through a tube into the neutral solution of bromothymol blue. Co2 h2o h2co3 h hco3 the h protonate the bromothymol blue which becomes yellow.
 Source: slideplayer.com
Source: slideplayer.com
Secondly what happens when bromothymol blue is added to a base. Secondly what happens when bromothymol blue is added to a base. Place bromothymol blue in a flask and use a straw to blow into the liquid. For drama make sure you put on safety goggles. Co2 h2o h2co3 h hco3 the h protonate the bromothymol blue which becomes yellow.
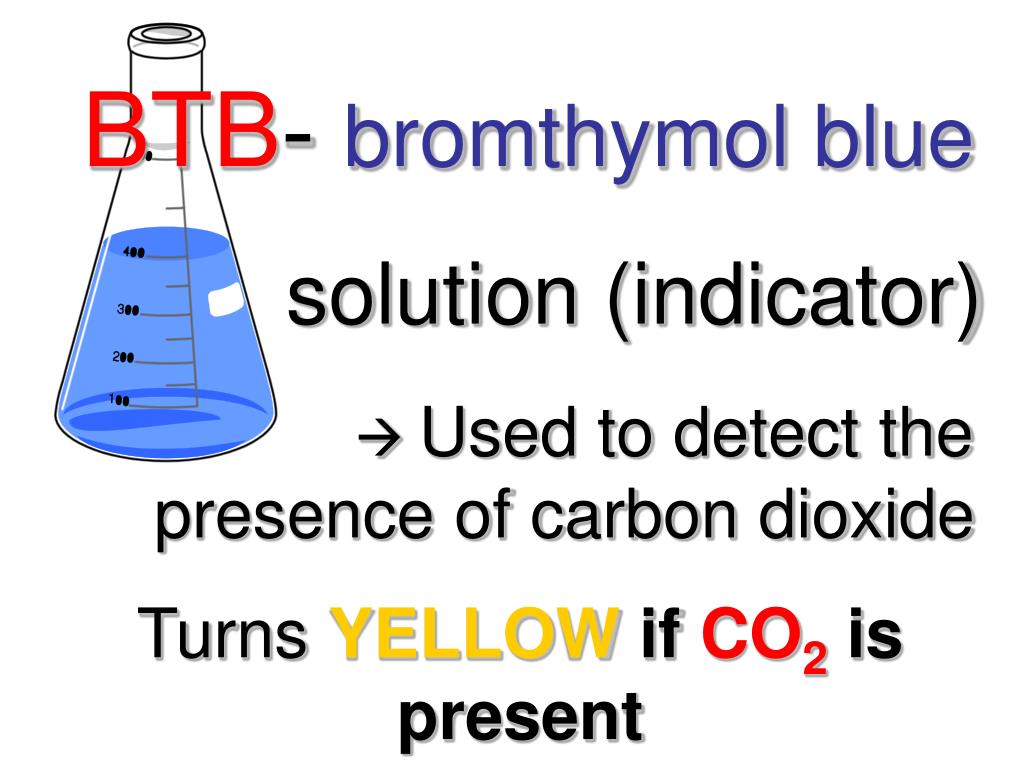 Source: slideserve.com
Source: slideserve.com
Bromthymol blue changes color over a ph range from 6 0 yellow to 7 6 blue. Bromothymol blue is a ph indicator. Co2 h2o h2co3 h hco3 the h protonate the bromothymol blue which becomes yellow. When it turns yellow it is overshot and. Secondly what happens when bromothymol blue is added to a base.
 Source: cobblearning.net
Source: cobblearning.net
A common demonstration of the ph indicator properties of btb involves exhaling through a tube into the neutral solution of bromothymol blue. For drama make sure you put on safety goggles. It will change colors i. Color may vary when you first open the container. Bromothymol blue is a ph indicator.
 Source: youtube.com
Source: youtube.com
A common use is for measuring the presence of carbonic acid in a liquid. For drama make sure you put on safety goggles. It is mostly used in applications that require measuring substances that would have a relatively neutral ph. Secondly what happens when bromothymol blue is added to a base. Bromothymol blue bmb is an indicator dye that turns yellow in the presence of acid.
 Source: m.youtube.com
Source: m.youtube.com
It will change colors i. Color may vary when you first open the container. Bromothymol blue is a ph indicator. A common use is for measuring the presence of carbonic acid in a liquid. The btb will turn yellow after you ve blown into it this change occurs because the carbon dioxide in your breath is acidic and btb is an indicator.
 Source: mimichem.weebly.com
Source: mimichem.weebly.com
It will change colors i. A common demonstration of the ph indicator properties of btb involves exhaling through a tube into the neutral solution of bromothymol blue. It is typically sold in solid form as the sodium salt of the acid indicator. We use bromothymal blue as an indicator to determine if the solution is acidic. Bromthymol blue indicates carbon dioxide is present.
 Source: pinterest.com
Source: pinterest.com
It will change colors i. It is typically sold in solid form as the sodium salt of the acid indicator. Bromthymol blue indicates carbon dioxide is present. Bromothymol blue bmb is an indicator dye that turns yellow in the presence of acid. When carbon dioxide is added to the solution it creates carbonic acid lowering the ph of the solution.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title bromothymol blue and carbon dioxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







