Bromothymol blue indicator preparation
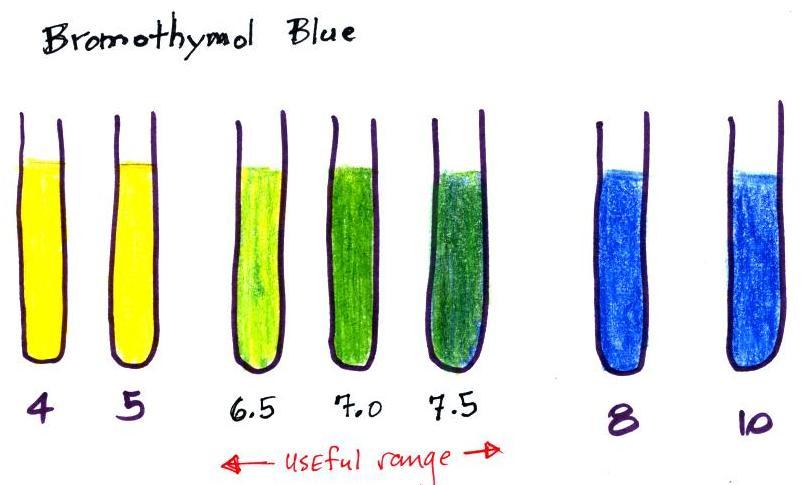
Bromothymol Blue Indicator Preparation. Bromothymol blue preparation bromothymol blue is a large compound with three benzene rings with two bromines where the bromo in the name comes from a sulfur attached to three oxygens a thym. Bromothymol blue acts as a weak acid in solution. Dissolve 50 mg of bromothymol blue in 4 ml of 0 02 m sodium hydroxide and 20 ml of ethanol 95 percent. Bromothymol blue is yellow in acidic solutions and blue in basic solutions.

Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. It contains a ph indicator bromothymol blue which causes the medium to change from yellow to blue green. To prepare a solution for use as ph indicator dissolve 0 10 g in 8 0 cm 3 n 50 naoh and dilute with water to 250 cm 3. Bromothymol blue also known as bromothymol sulfone phthalein and btb is a ph indicator. Bromothymol blue solution is used as an indicator to determine the rough ph of a substance. Bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity.
It is mostly used in applications that require measuring substances that would have a relatively neutral ph near 7.
Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. Aqueous bromothymol blue indicator solution. Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. Bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. To prepare a solution for use as ph indicator dissolve 0 10 g in 8 0 cm 3 n 50 naoh and dilute with water to 250 cm 3. Bromthymol blue is an indicator used to show the presence of an acidic solution.
 Source: atmo.arizona.edu
Source: atmo.arizona.edu
A common use is for measuring the presence of carbonic acid in a liquid 1. To prepare a solution for use as ph indicator dissolve 0 10 g in 8 0 cm 3 n 50 naoh and dilute with water to 250 cm 3. It is prepared from a powder household items and common laboratory chemicals that can be obtained through a scientific supply house individually or as a kit. The mixed bromothymol blue solution will turn yellow in an acidic. Reagecon s bromothymol blue r1 solution is a high quality indicator solution produced from the highest quality raw materials and manufactured and tested under rigorous conditions.
 Source: researchgate.net
Source: researchgate.net
To prepare a solution for use as ph indicator dissolve 0 10 g in 8 0 cm 3 n 50 naoh and dilute with water to 250 cm 3. Bromothymol blue preparation bromothymol blue is a large compound with three benzene rings with two bromines where the bromo in the name comes from a sulfur attached to three oxygens a thym. A common use is for measuring the presence of carbonic acid in a liquid 1. Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. The mixed bromothymol blue solution will turn yellow in an acidic.
 Source: study.com
Source: study.com
It contains antimicrobial agents chloramphenicol and chlortetracycline and an antimycotic agent cycloheximide. Bromothymol blue indicator solution. Bromthymol blue is an indicator used to show the presence of an acidic solution. Bromothymol blue is synthesized by addition of elemental bromine to thymol blue in a solution in glacial acetic acid. To prepare a solution for use as ph indicator dissolve 0 10 g in 8 0 cm 3 n 50 naoh and dilute with water to 250 cm 3.
 Source: alchetron.com
Source: alchetron.com
Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. Bromothymol blue solution is used as an indicator to determine the rough ph of a substance. It contains antimicrobial agents chloramphenicol and chlortetracycline and an antimycotic agent cycloheximide. Reagecon s bromothymol blue r1 solution is a high quality indicator solution produced from the highest quality raw materials and manufactured and tested under rigorous conditions. Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid.
 Source: alchetron.com
Source: alchetron.com
Dissolve 50 mg of bromothymol blue in 4 ml of 0 02 m sodium hydroxide and 20 ml of ethanol 95 percent. Bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. A common use is for measuring the presence of carbonic acid in a liquid 1. After the solution is effected add sufficient water to produce 100 ml. It is prepared from a powder household items and common laboratory chemicals that can be obtained through a scientific supply house individually or as a kit.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It contains antimicrobial agents chloramphenicol and chlortetracycline and an antimycotic agent cycloheximide. Reagecon s bromothymol blue r1 solution is a high quality indicator solution produced from the highest quality raw materials and manufactured and tested under rigorous conditions. The mixed bromothymol blue solution will turn yellow in an acidic. It contains antimicrobial agents chloramphenicol and chlortetracycline and an antimycotic agent cycloheximide. To prepare a solution that is used as a ph indicator we should dissolve 0 10 g in an 8 0 cm3 n 50 naoh and then dilute it with water to 250 cm3.
 Source: sciencedirect.com
Source: sciencedirect.com
Low levels of acid will result in the bromthymol blue solution remaining blue while higher levels of acid will result in the bromthymol blue solution taking on a yellow tint. Bromothymol blue preparation bromothymol blue is a large compound with three benzene rings with two bromines where the bromo in the name comes from a sulfur attached to three oxygens a thym. Bromothymol blue acts as a weak acid in solution. Aqueous bromothymol blue indicator solution. To prepare a solution that is used as a ph indicator we should dissolve 0 10 g in an 8 0 cm3 n 50 naoh and then dilute it with water to 250 cm3.
 Source: sciencecompany.com
Source: sciencecompany.com
After the solution is effected add sufficient water to produce 100 ml. Bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. Bromothymol blue acts as a weak acid in solution. It contains antimicrobial agents chloramphenicol and chlortetracycline and an antimycotic agent cycloheximide. Bromthymol blue is an indicator used to show the presence of an acidic solution.
 Source: m.youtube.com
Source: m.youtube.com
Bromothymol blue preparation bromothymol blue is a large compound with three benzene rings with two bromines where the bromo in the name comes from a sulfur attached to three oxygens a thym. Bromothymol blue indicator solution. Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid. Bromothymol blue solution is used as an indicator to determine the rough ph of a substance. Bromothymol blue btb bromothymol blue is an indicator a substance that changes color as the ph of a solution changes.

Indicator titrant or analyte change colour and this colour change is by far the most important method of end point detection in such titrations. It contains antimicrobial agents chloramphenicol and chlortetracycline and an antimycotic agent cycloheximide. Bromothymol blue btb bromothymol blue is an indicator a substance that changes color as the ph of a solution changes. A common use is for measuring the presence of carbonic acid in a liquid 1. Bromothymol blue acts as a weak acid in solution.
 Source: titrations.info
Source: titrations.info
The mixed bromothymol blue solution will turn yellow in an acidic. Low levels of acid will result in the bromthymol blue solution remaining blue while higher levels of acid will result in the bromthymol blue solution taking on a yellow tint. Dissolve 50 mg of bromothymol blue in 4 ml of 0 02 m sodium hydroxide and 20 ml of ethanol 95 percent. Reagecon s bromothymol blue r1 solution is a high quality indicator solution produced from the highest quality raw materials and manufactured and tested under rigorous conditions. Bromothymol blue is yellow in acidic solutions and blue in basic solutions.
 Source: thelivingtank.co.uk
Source: thelivingtank.co.uk
Bromthymol blue is an indicator used to show the presence of an acidic solution. To prepare a solution that is used as a ph indicator we should dissolve 0 10 g in an 8 0 cm3 n 50 naoh and then dilute it with water to 250 cm3. Bromothymol blue is synthesized by addition of elemental bromine to thymol blue in a solution in glacial acetic acid. Dissolve 50 mg of bromothymol blue in 4 ml of 0 02 m sodium hydroxide and 20 ml of ethanol 95 percent. The mixed bromothymol blue solution will turn yellow in an acidic.
 Source: sciencecompany.com
Source: sciencecompany.com
Indicator titrant or analyte change colour and this colour change is by far the most important method of end point detection in such titrations. It contains a ph indicator bromothymol blue which causes the medium to change from yellow to blue green. To prepare a solution for use as indicator in volumetric work dissolve 0 1 g in 100 cm 3 of 50 v v ethanol. Bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. After the solution is effected add sufficient water to produce 100 ml.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Bromothymol blue btb bromothymol blue is an indicator a substance that changes color as the ph of a solution changes. Bromothymol blue is the most commonly used ph indicator and is in low concentration and size container and low toxicity. Dissolve 50 mg of bromothymol blue in 4 ml of 0 02 m sodium hydroxide and 20 ml of ethanol 95 percent. Aqueous bromothymol blue indicator solution. Bromothymol blue preparation bromothymol blue is a large compound with three benzene rings with two bromines where the bromo in the name comes from a sulfur attached to three oxygens a thym.

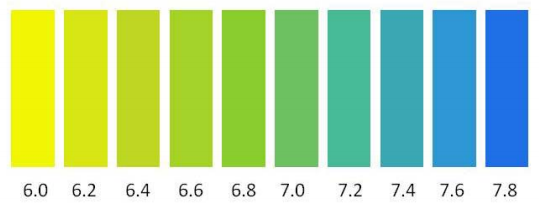
Dissolve 50 mg of bromothymol blue in 4 ml of 0 02 m sodium hydroxide and 20 ml of ethanol 95 percent. After the solution is effected add sufficient water to produce 100 ml. Bromothymol blue is an indicator in the ph range from 6 0 to 7 6. It contains a ph indicator bromothymol blue which causes the medium to change from yellow to blue green. Bromothymol blue is synthesized by addition of elemental bromine to thymol blue in a solution in glacial acetic acid.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title bromothymol blue indicator preparation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.