Chromatography definition for kids
Chromatography Definition For Kids. An example of a hpccc system. Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption. It consists of a stationary phase a solid and a mobile phase a liquid or a gas. In chemistry chromatography is a process for separating different components from a mixture.
 Types Of Chromatography Definition Principle Differential Extraction Faqs From byjus.com
Types Of Chromatography Definition Principle Differential Extraction Faqs From byjus.com
Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase. Chromatography is actually a way of separating out a mixture of chemicals which are in gas or liquid form by letting them creep slowly past another substance which is typically a liquid or solid. A process in which a chemical mixture carried by a liquid or gas is separated into components as a result of differential distribution of the solutes as they flow around or over a stationary liquid or solid phase. This is achieved by passing a sample mixture the analyte in a stream of solvent the mobile phase through some form of material the stationary phase that will provide resistance by virtue of chemical interactions not reactions between the components of the sample and the material. It consists of a stationary phase a solid and a mobile phase a liquid or a gas.
Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption.
This is achieved by passing a sample mixture the analyte in a stream of solvent the mobile phase through some form of material the stationary phase that will provide resistance by virtue of chemical interactions not reactions between the components of the sample and the material. Chromatography is much used in biochemistry and analytical chemistry. In chemistry chromatography is a process for separating different components from a mixture. The mixture is dissolved in a fluid gas solvent water called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which is fixed a material called the stationary phase. It consists of a stationary phase a solid and a mobile phase a liquid or a gas. Updated september 05 2019.
 Source: youtube.com
Source: youtube.com
Chromatography is a separation method that exploits the differences in partitioning behavior between a mobile phaseand a stationary phaseto separate the components in a mixture. The mixture is dissolved in a fluid gas solvent water called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which is fixed a material called the stationary phase. This is achieved by passing a sample mixture the analyte in a stream of solvent the mobile phase through some form of material the stationary phase that will provide resistance by virtue of chemical interactions not reactions between the components of the sample and the material. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. There are a variety of types of chromatography which can be used in.
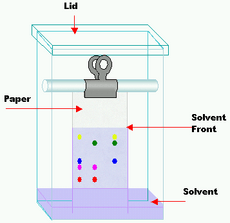 Source: kids.kiddle.co
Source: kids.kiddle.co
It consists of a stationary phase a solid and a mobile phase a liquid or a gas. A process in which a chemical mixture carried by a liquid or gas is separated into components as a result of differential distribution of the solutes as they flow around or over a stationary liquid or solid phase. There are a variety of types of chromatography which can be used in. Chromatography is a separation method that exploits the differences in partitioning behavior between a mobile phaseand a stationary phaseto separate the components in a mixture. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase.
 Source: education.com
Source: education.com
Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption. The mobile phase flows through the stationary phase. A process in which a chemical mixture carried by a liquid or gas is separated into components as a result of differential distribution of the solutes as they flow around or over a stationary liquid or solid phase. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase. An example of a hpccc system.
 Source: rainydaymum.co.uk
Source: rainydaymum.co.uk
The mixture is dissolved in a fluid gas solvent water called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which is fixed a material called the stationary phase. In chemistry chromatography is a process for separating different components from a mixture. Chromatography is a method using mixed substances that depends on the speed at which they move through special media or chemical substances. Chromatography is a laboratory technique for the separation of a mixture. Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption.
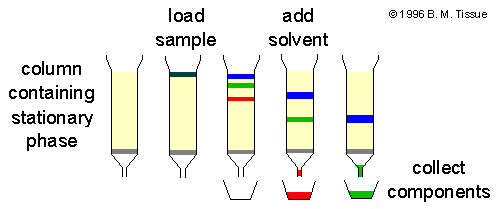 Source: chemicool.com
Source: chemicool.com
Chromatography is much used in biochemistry and analytical chemistry. Chromatography is a method using mixed substances that depends on the speed at which they move through special media or chemical substances. Chromatography is actually a way of separating out a mixture of chemicals which are in gas or liquid form by letting them creep slowly past another substance which is typically a liquid or solid. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase. Updated september 05 2019.
 Source: explainthatstuff.com
Source: explainthatstuff.com
Chromatography is a separation method that exploits the differences in partitioning behavior between a mobile phaseand a stationary phaseto separate the components in a mixture. A process in which a chemical mixture carried by a liquid or gas is separated into components as a result of differential distribution of the solutes as they flow around or over a stationary liquid or solid phase. Typically the sample is suspended in the liquid or gas phase and is separated or identified based on how it flows through or around a liquid or solid phase. Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption. An example of a hpccc system.
 Source: byjus.com
Source: byjus.com
Chromatography is actually a way of separating out a mixture of chemicals which are in gas or liquid form by letting them creep slowly past another substance which is typically a liquid or solid. Chromatography is much used in biochemistry and analytical chemistry. There are a variety of types of chromatography which can be used in. Updated september 05 2019. Chromatography is a method using mixed substances that depends on the speed at which they move through special media or chemical substances.
 Source: chemdictionary.org
Source: chemdictionary.org
Typically the sample is suspended in the liquid or gas phase and is separated or identified based on how it flows through or around a liquid or solid phase. Typically the sample is suspended in the liquid or gas phase and is separated or identified based on how it flows through or around a liquid or solid phase. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. Chromatography is a laboratory technique for the separation of a mixture.
 Source: explainthatstuff.com
Source: explainthatstuff.com
This is achieved by passing a sample mixture the analyte in a stream of solvent the mobile phase through some form of material the stationary phase that will provide resistance by virtue of chemical interactions not reactions between the components of the sample and the material. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. Chromatography is a laboratory technique for the separation of a mixture. An example of a hpccc system. Chromatography is a method using mixed substances that depends on the speed at which they move through special media or chemical substances.
 Source: youtube.com
Source: youtube.com
A process in which a chemical mixture carried by a liquid or gas is separated into components as a result of differential distribution of the solutes as they flow around or over a stationary liquid or solid phase. Chromatography is much used in biochemistry and analytical chemistry. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase. The mixture is dissolved in a fluid gas solvent water called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which is fixed a material called the stationary phase. Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption.
 Source: explainthatstuff.com
Source: explainthatstuff.com
Chromatography is a laboratory technique for the separation of a mixture. Chromatography is actually a way of separating out a mixture of chemicals which are in gas or liquid form by letting them creep slowly past another substance which is typically a liquid or solid. Chromatography is a group of laboratory techniques used to separate the components of a mixture by passing the mixture through a stationary phase. It consists of a stationary phase a solid and a mobile phase a liquid or a gas. Chromatography is a separation method that exploits the differences in partitioning behavior between a mobile phaseand a stationary phaseto separate the components in a mixture.
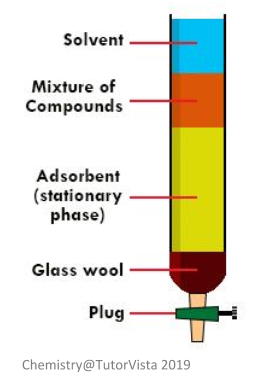 Source: alevelchemistry.co.uk
Source: alevelchemistry.co.uk
Components of a mixture may be interacting with the stationary phase based on charge relative solubility or adsorption. There are a variety of types of chromatography which can be used in. Chromatography is actually a way of separating out a mixture of chemicals which are in gas or liquid form by letting them creep slowly past another substance which is typically a liquid or solid. It consists of a stationary phase a solid and a mobile phase a liquid or a gas. The mixture is dissolved in a fluid gas solvent water called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which is fixed a material called the stationary phase.
 Source: study.com
Source: study.com
Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. It consists of a stationary phase a solid and a mobile phase a liquid or a gas. There are a variety of types of chromatography which can be used in. In chemistry chromatography is a process for separating different components from a mixture. A process in which a chemical mixture carried by a liquid or gas is separated into components as a result of differential distribution of the solutes as they flow around or over a stationary liquid or solid phase.
 Source: youtube.com
Source: youtube.com
The mobile phase flows through the stationary phase. This is achieved by passing a sample mixture the analyte in a stream of solvent the mobile phase through some form of material the stationary phase that will provide resistance by virtue of chemical interactions not reactions between the components of the sample and the material. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. An example of a hpccc system. Chromatography is a method using mixed substances that depends on the speed at which they move through special media or chemical substances.
 Source: byjus.com
Source: byjus.com
The mobile phase flows through the stationary phase. Chromatography is a laboratory technique for the separation of a mixture. Chromatography is a separation method that exploits the differences in partitioning behavior between a mobile phaseand a stationary phaseto separate the components in a mixture. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. The mixture is dissolved in a fluid gas solvent water called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which is fixed a material called the stationary phase.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title chromatography definition for kids by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.