Hand warmers reaction
Hand Warmers Reaction. I want to help you achieve the grades you and i know you are capable of. When the outer covering of a disposable hand warmer is removed air diffuses through the permeable pouch and reacts with the iron to form iron iii oxide. There are two common types of hand warmers one producing heat by the oxidation of a metal and the other by the crystallization of a salt. Max temperature of 158 degrees fahrenheit.
 Chemical Engineering Question Advanced Energy Bal Chegg Com From chegg.com
Chemical Engineering Question Advanced Energy Bal Chegg Com From chegg.com
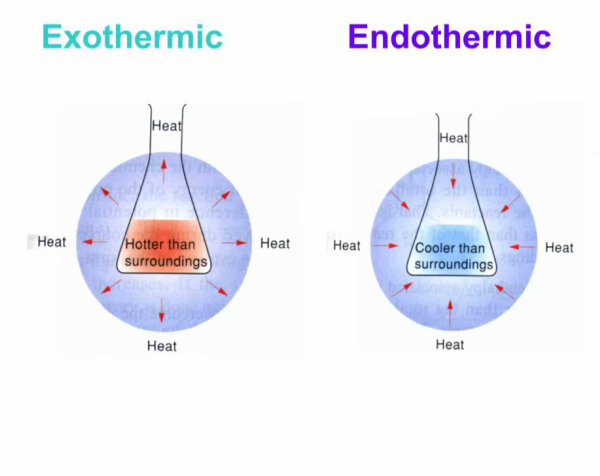
The iron within the hand warmer mixes with the oxygen in the air and oxides because it gives off heat when it reacts the process is known as an exothermic oxidation 4fe s 302 g 2fe203 s background research. The disposable hand warmer was invented in 1981 by japan s mycoal corporation which was then passed to their associate mycoal usa. There are two common types of hand warmers one producing heat by the oxidation of a metal and the other by the crystallization of a salt. Open the plastic packaging and air seeps in through the fabric pouch setting off the chemistry that heats things up. As the name suggests air activated hand warmers work when the hand warmer is removed from its package and exposed to air. By using iron filings in the hand warmer the exposed sides react.
There are two main types of chemical hand warmers.
The disposable hand warmer was invented in 1981 by japan s mycoal corporation which was then passed to their associate mycoal usa. Reaches average temperature of 135 degrees fahrenheit. There are two main types of chemical hand warmers. Even if you don t want to stud. The other type releases heat when a supersaturated solution crystallizes. One type releases heat by air activation.
 Source: slideplayer.com
Source: slideplayer.com
The disposable hand warmer was invented in 1981 by japan s mycoal corporation which was then passed to their associate mycoal usa. The air activated hand warmer generally contains iron powder water salt carbon and cellulose which all act together with the air to create a chemical reaction. This chemical reaction is very similar to the one that forms rust on your car and releases enough heat to warm your hands. One type releases heat by air activation. Even if you don t want to stud.
 Source: youtube.com
Source: youtube.com
For best results use hand warmer in an enclosed area with air such as a pocket or glove. Chemical hand warmers rely on exothermic chemical reactions to release heat. Reaches average temperature of 135 degrees fahrenheit. There are two main types of chemical hand warmers. For best results use hand warmer in an enclosed area with air such as a pocket or glove.
 Source: orvelleblog.blogspot.com
Source: orvelleblog.blogspot.com
The other type releases heat when a supersaturated solution crystallizes. This chemical reaction is very similar to the one that forms rust on your car and releases enough heat to warm your hands. As the name suggests air activated hand warmers work when the hand warmer is removed from its package and exposed to air. There are two main types of chemical hand warmers. Chemical solution hand warmers are re usable.
 Source: ourchemistryhandwarmer.weebly.com
Source: ourchemistryhandwarmer.weebly.com
The iron within the hand warmer mixes with the oxygen in the air and oxides because it gives off heat when it reacts the process is known as an exothermic oxidation 4fe s 302 g 2fe203 s background research. Open the plastic packaging and air seeps in through the fabric pouch setting off the chemistry that heats things up. This chemical reaction is very similar to the one that forms rust on your car and releases enough heat to warm your hands. One type releases heat by air activation. The hand warmer can be recharged by shaking or massaging the packet in air exposing more sides to the air to react.
 Source: slideplayer.com
Source: slideplayer.com
Air activated hand warmers are single use products. By using iron filings in the hand warmer the exposed sides react. I want to help you achieve the grades you and i know you are capable of. The air and the iron powder combine to form iron oxide with salt being used as a catalyst to speed up and intensify the reaction. Popular hand warmers rely on heat releasing chemical reactions also known as exothermic reactions.
 Source: bsclarified.wordpress.com
Source: bsclarified.wordpress.com
Chemical hand warmers rely on exothermic chemical reactions to release heat. The air and the iron powder combine to form iron oxide with salt being used as a catalyst to speed up and intensify the reaction. More pores mean more air so the pouch for toe warmers has more holes than the. The other type releases heat when a supersaturated solution crystallizes. View in hd this is a quick vid showing the exothermic crystallisation reaction or chemical reaction of a simple hand warmer.

The air activated hand warmer generally contains iron powder water salt carbon and cellulose which all act together with the air to create a chemical reaction. As the name suggests air activated hand warmers work when the hand warmer is removed from its package and exposed to air. When the outer covering of a disposable hand warmer is removed air diffuses through the permeable pouch and reacts with the iron to form iron iii oxide. The air activated hand warmer generally contains iron powder water salt carbon and cellulose which all act together with the air to create a chemical reaction. For best results use hand warmer in an enclosed area with air such as a pocket or glove.
 Source: youtube.com
Source: youtube.com
By using iron filings in the hand warmer the exposed sides react. Air activated hand warmers are single use products. One type releases heat by air activation. Chemical hand warmers rely on exothermic chemical reactions to release heat. Heating of the hand warmer.
 Source: outdoors.org
Source: outdoors.org
There are two main types of chemical hand warmers. Even if you don t want to stud. Air activated hand warmers are single use products. One type releases heat by air activation. I want to help you achieve the grades you and i know you are capable of.
 Source: chegg.com
Source: chegg.com
Open the plastic packaging and air seeps in through the fabric pouch setting off the chemistry that heats things up. The disposable hand warmer was invented in 1981 by japan s mycoal corporation which was then passed to their associate mycoal usa. When the outer covering of a disposable hand warmer is removed air diffuses through the permeable pouch and reacts with the iron to form iron iii oxide. Max temperature of 158 degrees fahrenheit. The air activated hand warmer generally contains iron powder water salt carbon and cellulose which all act together with the air to create a chemical reaction.
 Source: slide-finder.com
Source: slide-finder.com
The hand warmer can be recharged by shaking or massaging the packet in air exposing more sides to the air to react. There are two main types of chemical hand warmers. View in hd this is a quick vid showing the exothermic crystallisation reaction or chemical reaction of a simple hand warmer. Popular hand warmers rely on heat releasing chemical reactions also known as exothermic reactions. By using iron filings in the hand warmer the exposed sides react.
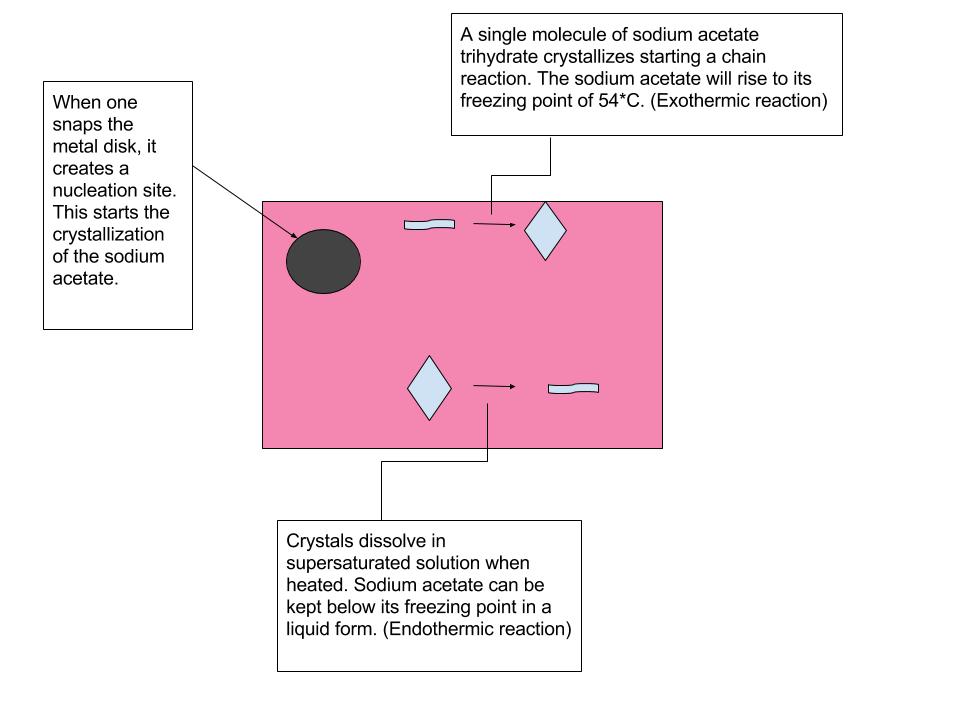 Source: emiliebaxter.weebly.com
Source: emiliebaxter.weebly.com
For best results use hand warmer in an enclosed area with air such as a pocket or glove. The other type releases heat when a supersaturated solution crystallizes. Reaches average temperature of 135 degrees fahrenheit. The air activated hand warmer generally contains iron powder water salt carbon and cellulose which all act together with the air to create a chemical reaction. The air and the iron powder combine to form iron oxide with salt being used as a catalyst to speed up and intensify the reaction.
 Source: m.youtube.com
Source: m.youtube.com
The air activated hand warmer generally contains iron powder water salt carbon and cellulose which all act together with the air to create a chemical reaction. Heating of the hand warmer. I want to help you achieve the grades you and i know you are capable of. Chemical solution hand warmers are re usable. The formation of the new compound releases energy into the surroundings which we feel as heat in the hand warmer reaction.
 Source: wired.com
Source: wired.com
There are two common types of hand warmers one producing heat by the oxidation of a metal and the other by the crystallization of a salt. There are two common types of hand warmers one producing heat by the oxidation of a metal and the other by the crystallization of a salt. Reaches average temperature of 135 degrees fahrenheit. One type releases heat by air activation. Max temperature of 158 degrees fahrenheit.
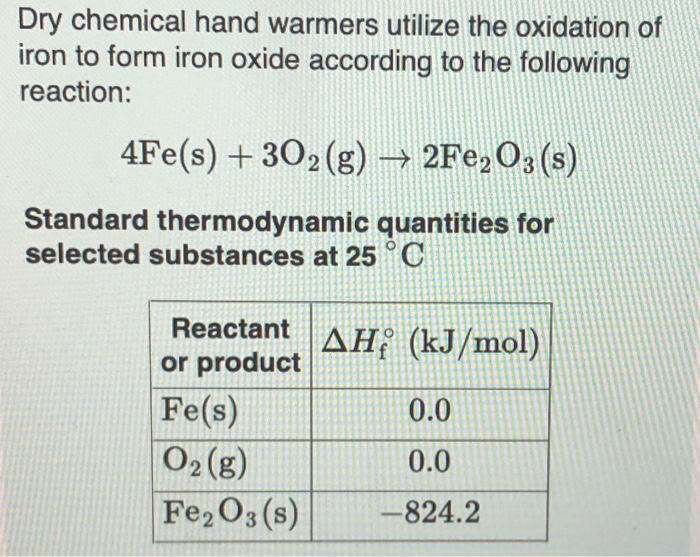 Source: chegg.com
Source: chegg.com
Even if you don t want to stud. More pores mean more air so the pouch for toe warmers has more holes than the. This chemical reaction is very similar to the one that forms rust on your car and releases enough heat to warm your hands. Popular hand warmers rely on heat releasing chemical reactions also known as exothermic reactions. The hand warmer can be recharged by shaking or massaging the packet in air exposing more sides to the air to react.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hand warmers reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.