Hot air balloon gas law
Hot Air Balloon Gas Law. Types of problems included. Charles s law says that the volume of a gas is directly related to the temperature of that gas similarly when a gas is heated like a burner in a hot air balloon the gas expands. In a hot air balloon the air that is heated is not in a rigid container but in a balloon that. How will the pressure of molecules outside the balloon effect the pressure of molecule inside the balloon.
 Combined Gas Law Ck 12 Foundation From flexbooks.ck12.org
Combined Gas Law Ck 12 Foundation From flexbooks.ck12.org
We know from the ideal gas laws that when you heat a gas under constant pressure it will expand. A hot air balloon works with the charle s law. When you fill the balloon with hot air it will eventually fill up all the way. The law that explains how hot air balloons work is the charles s law. Boyles law charles law gay lussac s law combined gas law ideal gas law included in this product. Charles law defines a relationship between the volume and the temperature of a gas.
Student handout with problems and multiple choice answers color by number hot air balloon handout for students to color with correct answers completed color by number fish key key with all problems completely worked.
So when the air inside the balloon expands it becomes less dense and provides the lift for the hot air balloon. So when the air inside the balloon expands it becomes less dense and provides the lift for the hot air balloon. If gas expands when it is heated a given weight of hot air occupies a larger volume then the same weight of cold. We know from the ideal gas laws that when you heat a gas under constant pressure it will expand. As the air in the balloon is heated by a small flame the balloon expands becoming less dense than the surrounding cool air. When you fill the balloon with hot air it will eventually fill up all the way.
 Source: prezi.com
Source: prezi.com
The rising of hot air balloons up in the air is an example of charles law. Unlike a party balloon a hot air balloon is not a closed system but open at the bottom where the gas burner heats the air and also open at the top more about this later. So when the air inside the balloon expands it becomes less dense and provides the lift for the hot air balloon. Boyles law charles law gay lussac s law combined gas law ideal gas law included in this product. The balloon thus rises.
 Source: youtube.com
Source: youtube.com
Types of problems included. The hot air balloon operates on the principle of charles s law which states that the volume of a gas increases with temperature. Charles s law says that the volume of a gas is directly related to the temperature of that gas similarly when a gas is heated like a burner in a hot air balloon the gas expands. If gas expands when it is heated a given weight of hot air occupies a larger volume then the same weight of cold. Boyles law charles law gay lussac s law combined gas law ideal gas law included in this product.
 Source: storyboardthat.com
Source: storyboardthat.com
The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. Charles s law says that the volume of a gas is directly related to the temperature of that gas similarly when a gas is heated like a burner in a hot air balloon the gas expands. As the air in the balloon is heated by a small flame the balloon expands becoming less dense than the surrounding cool air. The law that explains how hot air balloons work is the charles s law. Usually in examples of boyle s law our teachers mention hot air balloon that the size of balloon increases as pressure decreases.
 Source: socratic.org
Source: socratic.org
The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. If gas expands when it is heated a given weight of hot air occupies a larger volume then the same weight of cold. The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. When you fill the balloon with hot air it will eventually fill up all the way. Usually in examples of boyle s law our teachers mention hot air balloon that the size of balloon increases as pressure decreases.
 Source: letstalkscience.ca
Source: letstalkscience.ca
Usually in examples of boyle s law our teachers mention hot air balloon that the size of balloon increases as pressure decreases. But at as height increases pressure decreases because there are less molecules above us. How will the pressure of molecules outside the balloon effect the pressure of molecule inside the balloon. When you fill the balloon with hot air it will eventually fill up all the way. The law that explains how hot air balloons work is the charles s law.
 Source: flexbooks.ck12.org
Source: flexbooks.ck12.org
A hot air balloon works with the charle s law. Charles s law says that the volume of a gas is directly related to the temperature of that gas similarly when a gas is heated like a burner in a hot air balloon the gas expands. The law that explains how hot air balloons work is the charles s law. We know from the ideal gas laws that when you heat a gas under constant pressure it will expand. The hot air balloon operates on the principle of charles s law which states that the volume of a gas increases with temperature.
 Source: teacherspayteachers.com
Source: teacherspayteachers.com
The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. How will the pressure of molecules outside the balloon effect the pressure of molecule inside the balloon. In a hot air balloon the air that is heated is not in a rigid container but in a balloon that. Boyles law charles law gay lussac s law combined gas law ideal gas law included in this product. A hot air balloon works with the charle s law.
Source: quora.com
Charles s law says that the volume of a gas is directly related to the temperature of that gas similarly when a gas is heated like a burner in a hot air balloon the gas expands. In a hot air balloon the air that is heated is not in a rigid container but in a balloon that. The balloon thus rises. Charles law defines a relationship between the volume and the temperature of a gas. The law that explains how hot air balloons work is the charles s law.
 Source: sites.google.com
Source: sites.google.com
If gas expands when it is heated a given weight of hot air occupies a larger volume then the same weight of cold. Boyles law charles law gay lussac s law combined gas law ideal gas law included in this product. The balloon thus rises. Usually in examples of boyle s law our teachers mention hot air balloon that the size of balloon increases as pressure decreases. We know from the ideal gas laws that when you heat a gas under constant pressure it will expand.
 Source: prezi.com
Source: prezi.com
Charles law defines a relationship between the volume and the temperature of a gas. In a hot air balloon the air that is heated is not in a rigid container but in a balloon that. The balloon thus rises. The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. How will the pressure of molecules outside the balloon effect the pressure of molecule inside the balloon.
 Source: slideplayer.com
Source: slideplayer.com
The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. The balloon thus rises. Types of problems included. Now when you keep heating the air and you raise the temperature this means that the volume will have to increase as well. The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon.
 Source: chemistrygod.com
Source: chemistrygod.com
The hot air balloon operates on the principle of charles s law which states that the volume of a gas increases with temperature. The hot air balloon operates on the principle of charles s law which states that the volume of a gas increases with temperature. The decrease of the air mass inside the balloon can be explained by the fact that the heated air expands and partly flows out of the balloon. In a hot air balloon the air that is heated is not in a rigid container but in a balloon that. Student handout with problems and multiple choice answers color by number hot air balloon handout for students to color with correct answers completed color by number fish key key with all problems completely worked.
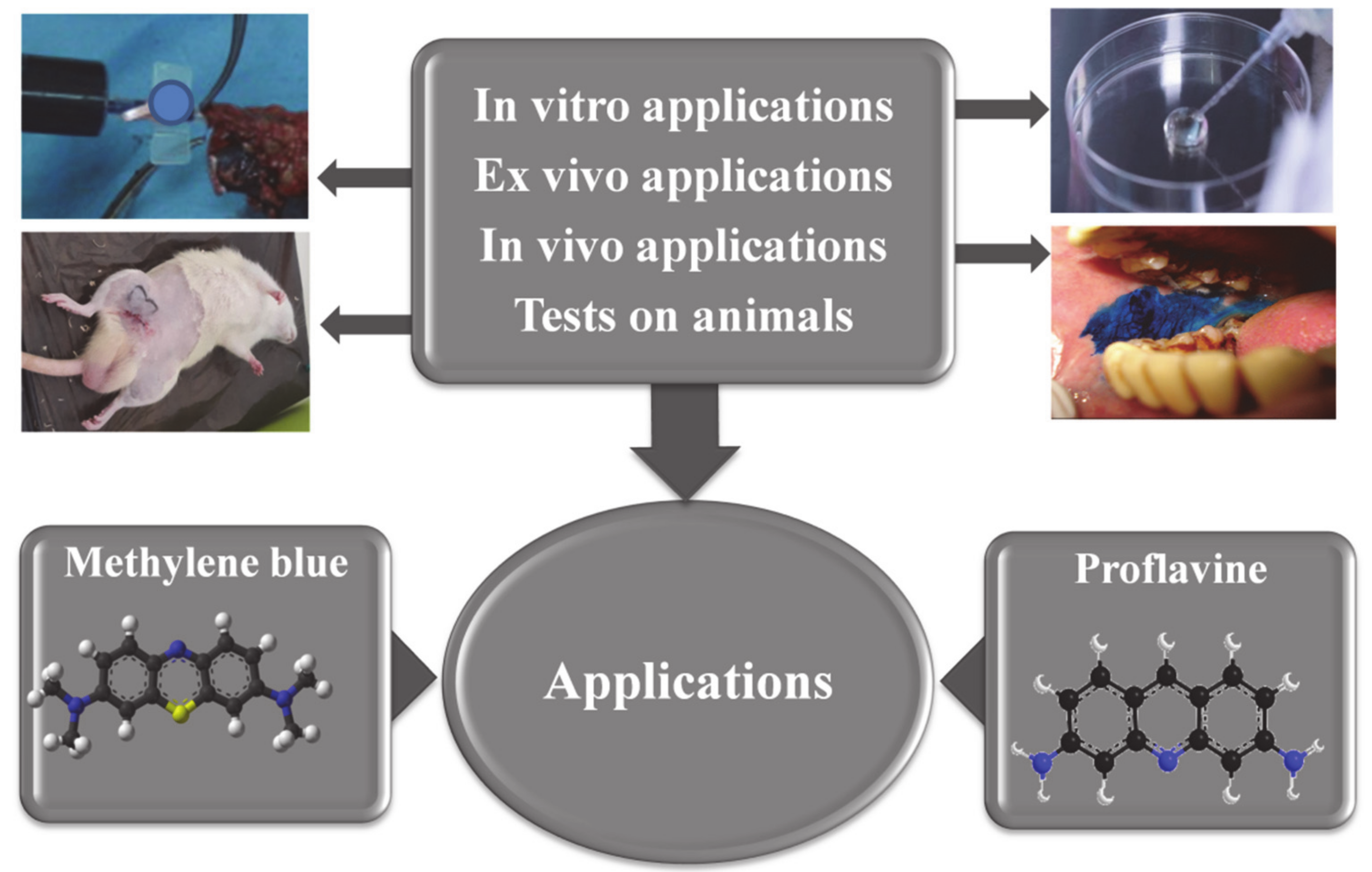
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
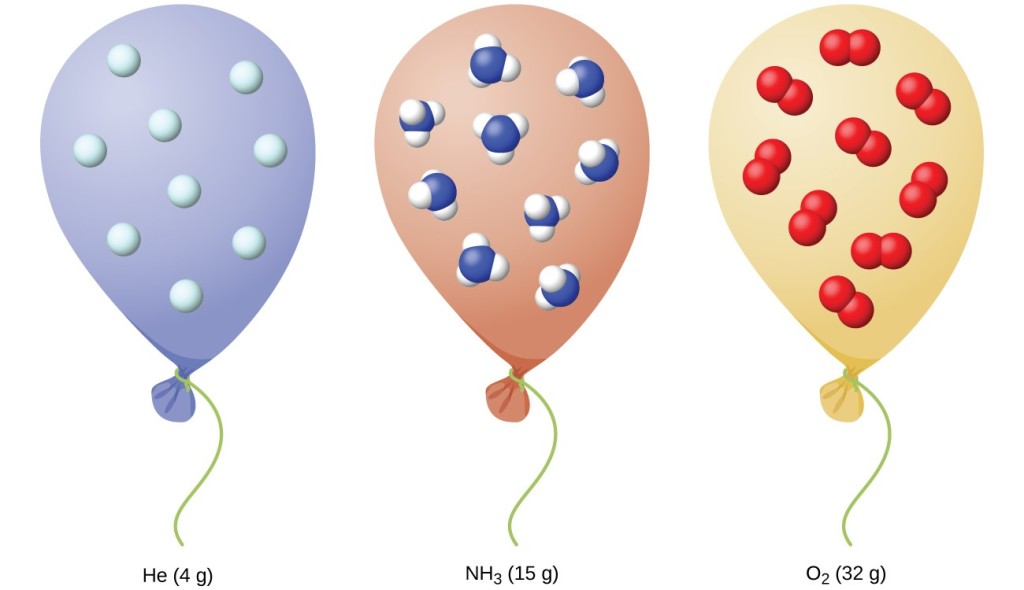
When you fill the balloon with hot air it will eventually fill up all the way. So when the air inside the balloon expands it becomes less dense and provides the lift for the hot air balloon. We know from the ideal gas laws that when you heat a gas under constant pressure it will expand. Types of problems included. In a hot air balloon the air that is heated is not in a rigid container but in a balloon that.
 Source: prezi.com
Source: prezi.com
How will the pressure of molecules outside the balloon effect the pressure of molecule inside the balloon. A hot air balloon works with the charle s law. We know from the ideal gas laws that when you heat a gas under constant pressure it will expand. Charles law defines a relationship between the volume and the temperature of a gas. Usually in examples of boyle s law our teachers mention hot air balloon that the size of balloon increases as pressure decreases.
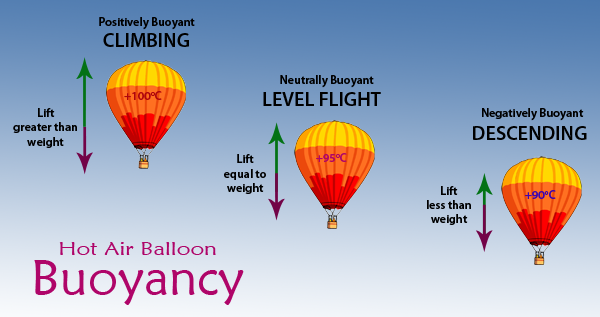 Source: brisbanehotairballooning.com.au
Source: brisbanehotairballooning.com.au
As the air in the balloon is heated by a small flame the balloon expands becoming less dense than the surrounding cool air. But at as height increases pressure decreases because there are less molecules above us. Unlike a party balloon a hot air balloon is not a closed system but open at the bottom where the gas burner heats the air and also open at the top more about this later. When you fill the balloon with hot air it will eventually fill up all the way. So when the air inside the balloon expands it becomes less dense and provides the lift for the hot air balloon.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hot air balloon gas law by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.