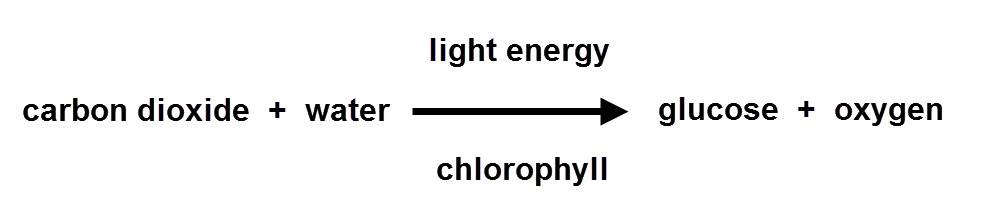
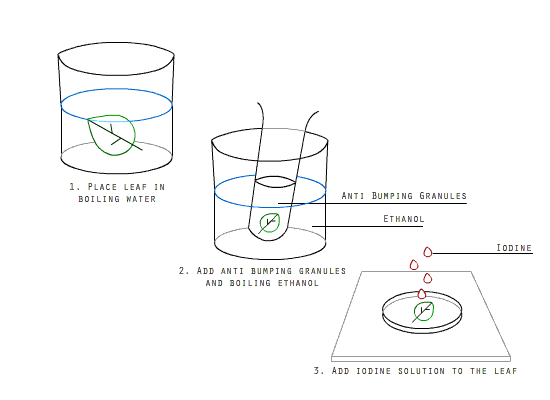
How is lime water made from calcium carbonate
How Is Lime Water Made From Calcium Carbonate. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic. This reaction creates a solid particle that sinks to the ocean floor. Bubbling carbon dioxide through this forms a milky. Initially the lime water is alkaline.
 British Lime Association Bla Part Of The Mineral Products Association Mpa From britishlime.org
British Lime Association Bla Part Of The Mineral Products Association Mpa From britishlime.org
1 heat strongly this will give you quick lime 2 add a few drops of water which will give you slaked lime which is. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration. Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour. That means it is made up of calcium carbon and oxygen. Its chemical symbol is caco3.
Lime water reacts with carbon dioxide to give calcium carbonate which forms an the calcium oxide unslaked lime is dissolved in water to form calcium hydroxide limewater.
Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration. It precipitates out as a white suspended solid making the solution appear cloudy. Bubbling carbon dioxide through this forms a milky. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste.
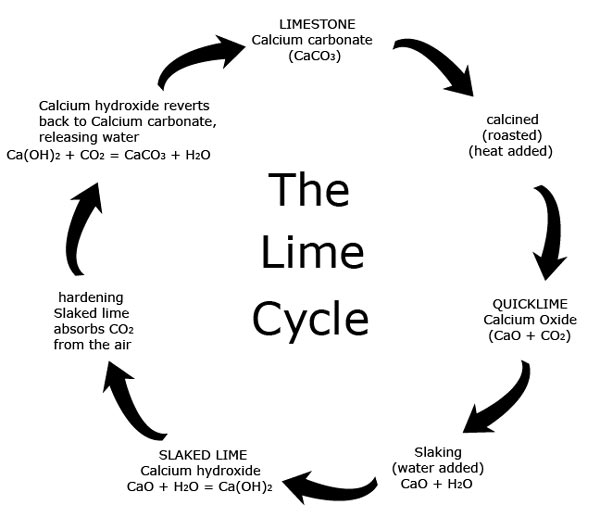 Source: dyfedarchaeology.org.uk
Source: dyfedarchaeology.org.uk
To produce this from caco3 heat the caco3 to decompose it this will require high temperature. If we bubble carbon dioxide or a mixture of gases which includes carbon dioxide such as air or our breath through lime water calcium carbonate is formed in suspension and the lime water goes milky. Lime what is calcium carbonate. When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced. Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. The process by which limestone calcium carbonate is converted to quicklime by heating then to slaked lime by hydration and naturally reverts to calcium carbonate by carbonation is called the lime cycle. This reaction creates a solid particle that sinks to the ocean floor. To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic.
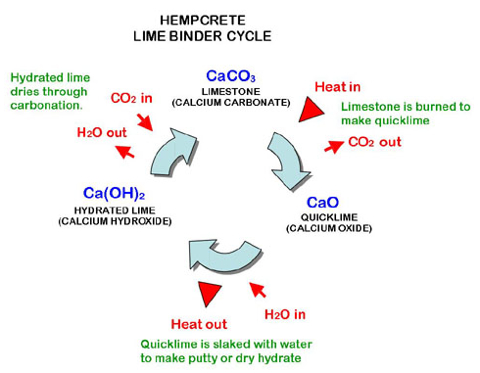 Source: hemptechglobal.com
Source: hemptechglobal.com
To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour. Lime stone is mainly made up of calcium carbonate to get to lime water. Lime what is calcium carbonate. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration. Initially the lime water is alkaline.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced. To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour. Initially the lime water is alkaline. If we bubble carbon dioxide or a mixture of gases which includes carbon dioxide such as air or our breath through lime water calcium carbonate is formed in suspension and the lime water goes milky. Lime water reacts with carbon dioxide to give calcium carbonate which forms an the calcium oxide unslaked lime is dissolved in water to form calcium hydroxide limewater.
Source: researchgate.net
It precipitates out as a white suspended solid making the solution appear cloudy. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste. That means it is made up of calcium carbon and oxygen. Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c.
 Source: oldbuilders.com
Source: oldbuilders.com
Initially the lime water is alkaline. To produce this from caco3 heat the caco3 to decompose it this will require high temperature. When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced. Lime what is calcium carbonate. Bubbling carbon dioxide through this forms a milky.
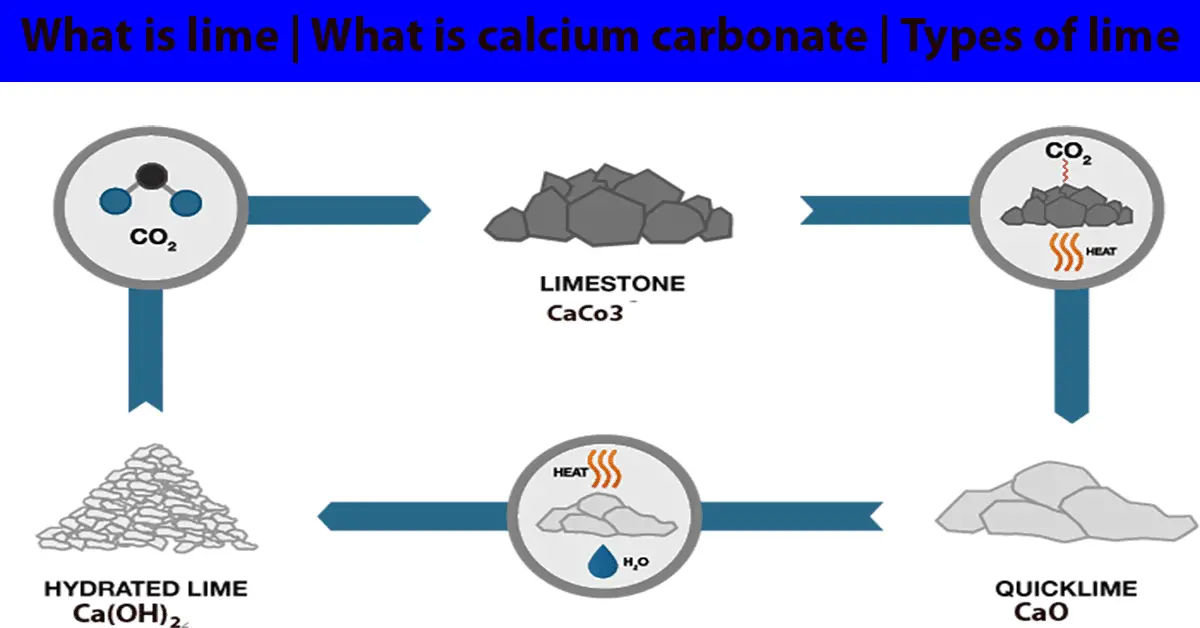 Source: civilquery.com
Source: civilquery.com
Lime stone is mainly made up of calcium carbonate to get to lime water. Its chemical symbol is caco3. 1 heat strongly this will give you quick lime 2 add a few drops of water which will give you slaked lime which is. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic.
 Source: slideplayer.com
Source: slideplayer.com
This reaction creates a solid particle that sinks to the ocean floor. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic. Lime what is calcium carbonate. 1 heat strongly this will give you quick lime 2 add a few drops of water which will give you slaked lime which is. Its chemical symbol is caco3.
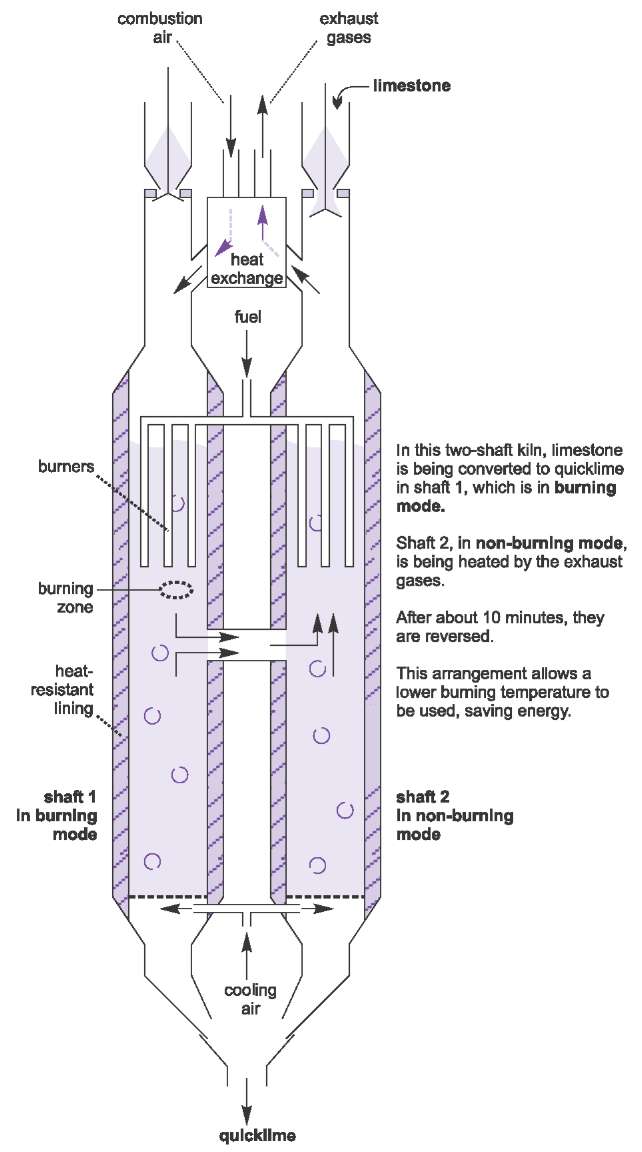 Source: essentialchemicalindustry.org
Source: essentialchemicalindustry.org
As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic. The process by which limestone calcium carbonate is converted to quicklime by heating then to slaked lime by hydration and naturally reverts to calcium carbonate by carbonation is called the lime cycle. Its chemical symbol is caco3. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste. When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced.
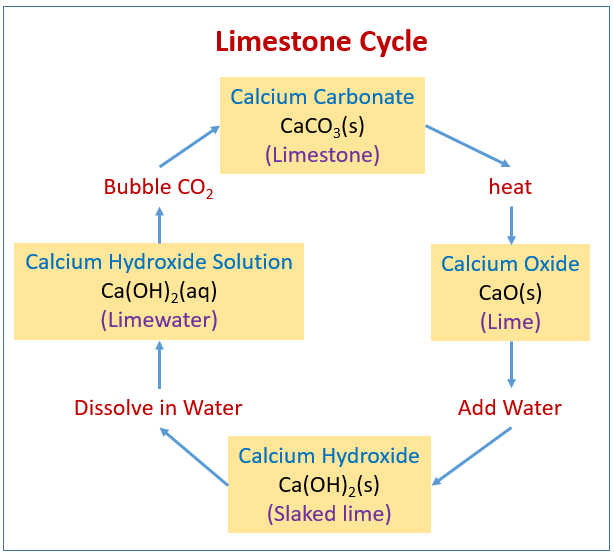 Source: onlinemathlearning.com
Source: onlinemathlearning.com
The process by which limestone calcium carbonate is converted to quicklime by heating then to slaked lime by hydration and naturally reverts to calcium carbonate by carbonation is called the lime cycle. 1 heat strongly this will give you quick lime 2 add a few drops of water which will give you slaked lime which is. Lime what is calcium carbonate. Lime stone is mainly made up of calcium carbonate to get to lime water. Lime water reacts with carbon dioxide to give calcium carbonate which forms an the calcium oxide unslaked lime is dissolved in water to form calcium hydroxide limewater.
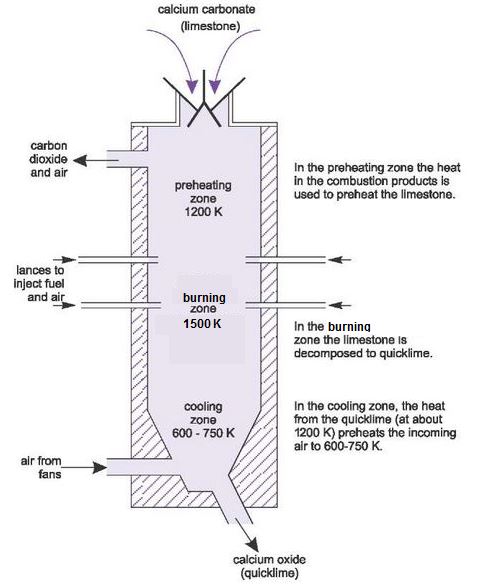 Source: essentialchemicalindustry.org
Source: essentialchemicalindustry.org
Lime water is a solution of calcium hydroxide. Its chemical symbol is caco3. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration. When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced. The process by which limestone calcium carbonate is converted to quicklime by heating then to slaked lime by hydration and naturally reverts to calcium carbonate by carbonation is called the lime cycle.
 Source: britishlime.org
Source: britishlime.org
Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringent bitter taste. It precipitates out as a white suspended solid making the solution appear cloudy. When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced. This reaction creates a solid particle that sinks to the ocean floor.
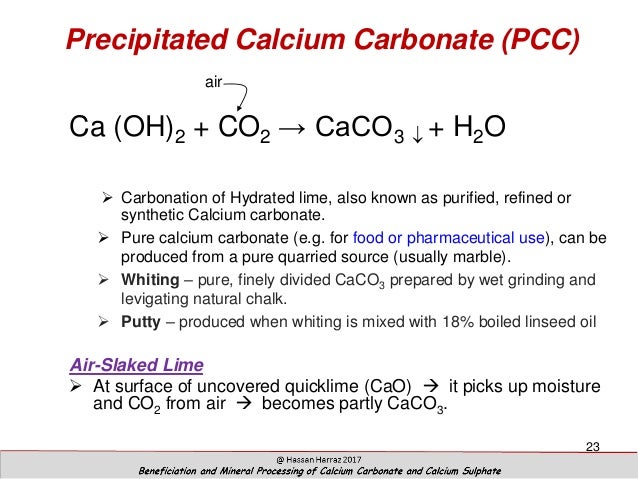 Source: slideshare.net
Source: slideshare.net
The process by which limestone calcium carbonate is converted to quicklime by heating then to slaked lime by hydration and naturally reverts to calcium carbonate by carbonation is called the lime cycle. Lime water is a solution of calcium hydroxide. It precipitates out as a white suspended solid making the solution appear cloudy. If we bubble carbon dioxide or a mixture of gases which includes carbon dioxide such as air or our breath through lime water calcium carbonate is formed in suspension and the lime water goes milky. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic.
Source: fao.org
Limewater is the common name for a dilute aqueous solution of calcium hydroxide calcium hydroxide ca oh 2 is sparsely soluble at room temperature in water 1 5 g l at 25 c. That means it is made up of calcium carbon and oxygen. When carbon dioxide is bubbled into limewater calcium carbonate caco 3 is produced. Lime stone is mainly made up of calcium carbonate to get to lime water. To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour.
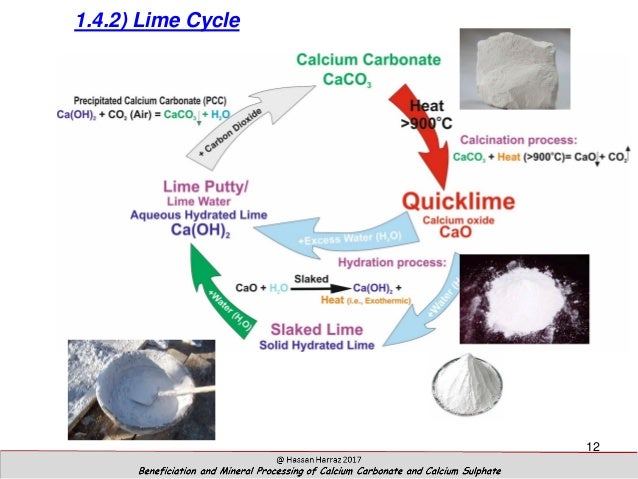 Source: slideshare.net
Source: slideshare.net
Lime water is a solution of calcium hydroxide. Lime what is calcium carbonate. Bubbling carbon dioxide through this forms a milky. To produce this from caco3 heat the caco3 to decompose it this will require high temperature. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how is lime water made from calcium carbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.