How to make lime water from calcium carbonate
How To Make Lime Water From Calcium Carbonate. Calcium carbonate is a compound that results from calcium ions reacting with carbonate ions in the water. To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour. This reaction creates a solid particle that sinks to the ocean floor. Add about 2 cm lime water to a test tube.
 Different Steps In The Making Of Eggshell Lime Download Scientific Diagram From researchgate.net
Different Steps In The Making Of Eggshell Lime Download Scientific Diagram From researchgate.net
Blow through a drinking straw to bubble co 2 through the lime water solution until it goes cloudy 20 30 seconds. After it is apparent that the rock has been cooked and decomposed turn the heat off. Heat your calcium carbonate directly on the flame until it becomes red hot. Decant the clear solution into a beaker. It is made by mixing calcium hydroxide in water 4 to 8 times the quantity of lime. Limewater is a saturated solution which means there will be some extra chemical that doesn t dissolve.
Lime water it reacts with the calcium hydroxide to produce calcium carbonate the calcium carbonate water runs down and eventually reaches an air filled to form carbon dioxide and lime an important material in making steel glass and during a process called calcination calcium carbonate limestone is burned and.
The rock will decompose on heating to create calcium oxide quicklime and carbon dioxide. Limewater can be made by dissolving either calcium oxide cao or calcium hydroxide ca oh 2 in water 6 when cao is used it first hydrates to ca oh 2 on contact with water h2o you can obtain. Turn the lime water cloudy. Lime what is calcium carbonate. That means it is made up of calcium carbon and oxygen. Add about 2 cm lime water to a test tube.
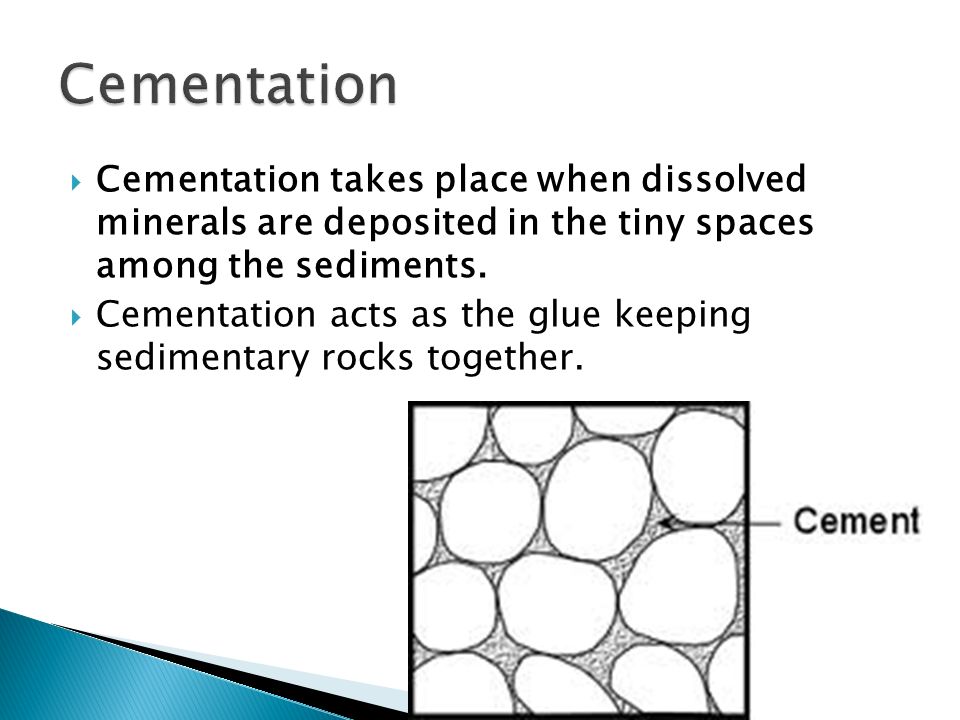
 Source: hemptechglobal.com
Source: hemptechglobal.com
The term can be used to refer to water that contains dissolved lime or calcium salts. Decant the clear solution into a beaker. Its chemical symbol is caco3. After it is apparent that the rock has been cooked and decomposed turn the heat off. The rock will decompose on heating to create calcium oxide quicklime and carbon dioxide.
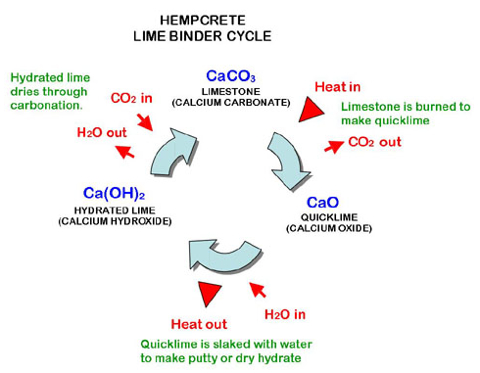
 Source: essentialchemicalindustry.org
Source: essentialchemicalindustry.org
Decant the clear solution into a beaker. Its chemical symbol is caco3. Blow through a drinking straw to bubble co 2 through the lime water solution until it goes cloudy 20 30 seconds. Lime water it reacts with the calcium hydroxide to produce calcium carbonate the calcium carbonate water runs down and eventually reaches an air filled to form carbon dioxide and lime an important material in making steel glass and during a process called calcination calcium carbonate limestone is burned and. After it is apparent that the rock has been cooked and decomposed turn the heat off.
 Source: education.com
Source: education.com
Add about 2 cm lime water to a test tube. It is made by mixing calcium hydroxide in water 4 to 8 times the quantity of lime. The rock will decompose on heating to create calcium oxide quicklime and carbon dioxide. Do this for about 2 3 minutes. Its chemical symbol is caco3.
 Source: superprof.co.uk
Source: superprof.co.uk
To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration. How to make limewater solution. Lime water it reacts with the calcium hydroxide to produce calcium carbonate the calcium carbonate water runs down and eventually reaches an air filled to form carbon dioxide and lime an important material in making steel glass and during a process called calcination calcium carbonate limestone is burned and. Put 1 teaspoon of calcium hydroxide in a clean glass jar up to 1 gallon in size. The rock will decompose on heating to create calcium oxide quicklime and carbon dioxide.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
The rock will decompose on heating to create calcium oxide quicklime and carbon dioxide. Lime water it reacts with the calcium hydroxide to produce calcium carbonate the calcium carbonate water runs down and eventually reaches an air filled to form carbon dioxide and lime an important material in making steel glass and during a process called calcination calcium carbonate limestone is burned and. That means it is made up of calcium carbon and oxygen. A teaspoon will result in a fully saturated solution whether you use a gallon jar or a smaller one. Limewater is a saturated solution which means there will be some extra chemical that doesn t dissolve.
 Source: researchgate.net
Source: researchgate.net
Turn the lime water cloudy. In other words limewater is a clear colorless aqueous solution of calcium hydroxide. Lime water it reacts with the calcium hydroxide to produce calcium carbonate the calcium carbonate water runs down and eventually reaches an air filled to form carbon dioxide and lime an important material in making steel glass and during a process called calcination calcium carbonate limestone is burned and. Lime what is calcium carbonate. How to make limewater solution.
 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
After it is apparent that the rock has been cooked and decomposed turn the heat off. Add about 2 cm lime water to a test tube. To convert calcium carbonate into calcium oxide you will need to heat the powdered calcium carbonate to 1000 c in a furnace for one hour. Limewater is a saturated solution which means there will be some extra chemical that doesn t dissolve. Blow through a drinking straw to bubble co 2 through the lime water solution until it goes cloudy 20 30 seconds.
 Source: sciencestruck.com
Source: sciencestruck.com
Decant the clear solution into a beaker. From there organisms can use it to build their shells. After it is apparent that the rock has been cooked and decomposed turn the heat off. The term can be used to refer to water that contains dissolved lime or calcium salts. Lime what is calcium carbonate.
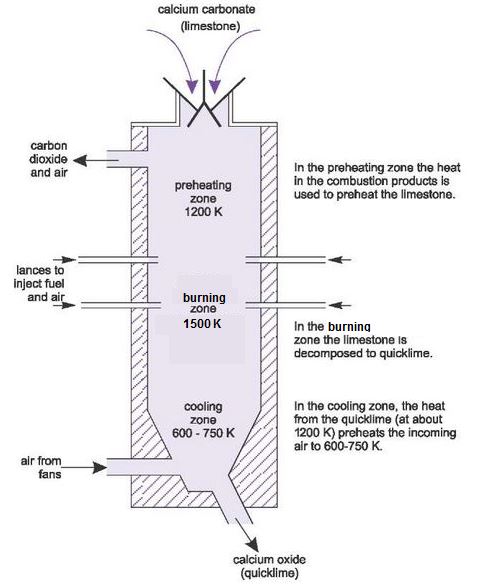
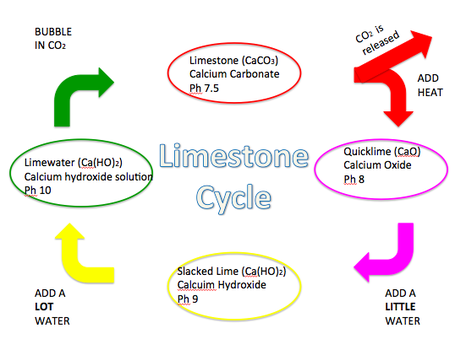 Source: educatingberkshire.weebly.com
Source: educatingberkshire.weebly.com
From there organisms can use it to build their shells. How to make limewater solution. After it is apparent that the rock has been cooked and decomposed turn the heat off. Blow through a drinking straw to bubble co 2 through the lime water solution until it goes cloudy 20 30 seconds. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration.
 Source: youtube.com
Source: youtube.com
Add about 2 cm lime water to a test tube. Lime what is calcium carbonate. How to make limewater solution. Decant the clear solution into a beaker. Put 1 teaspoon of calcium hydroxide in a clean glass jar up to 1 gallon in size.
 Source: quizlet.com
Source: quizlet.com
That means it is made up of calcium carbon and oxygen. Calcium carbonate is a compound that results from calcium ions reacting with carbonate ions in the water. After it is apparent that the rock has been cooked and decomposed turn the heat off. The rock will decompose on heating to create calcium oxide quicklime and carbon dioxide. Limewater is a saturated solution which means there will be some extra chemical that doesn t dissolve.
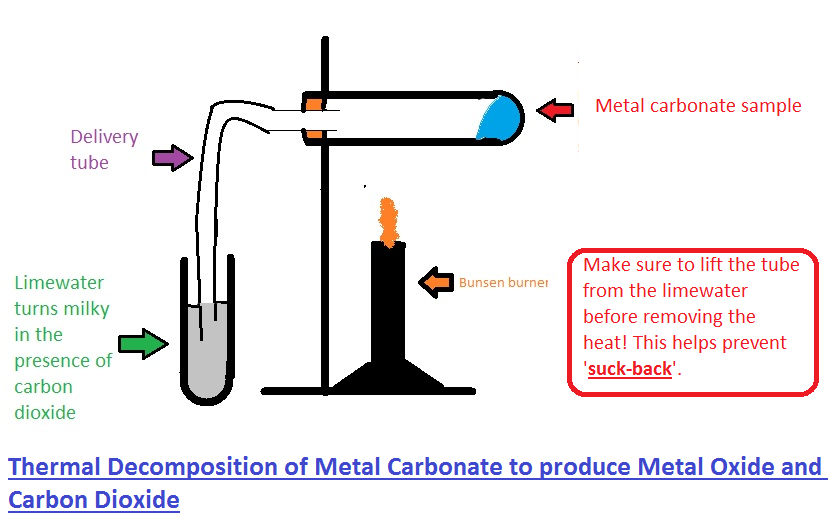 Source: goconqr.com
Source: goconqr.com
It is made by mixing calcium hydroxide in water 4 to 8 times the quantity of lime. Do this for about 2 3 minutes. To make lime water from calcium carbonate you first need to convert the calcium carbonate into calcium oxide and then convert the calcium oxide into calcium hydroxide by reaction with water hydration. A teaspoon will result in a fully saturated solution whether you use a gallon jar or a smaller one. It is made by mixing calcium hydroxide in water 4 to 8 times the quantity of lime.
 Source: igcse-chemistry-edexcel.blogspot.com
Source: igcse-chemistry-edexcel.blogspot.com
Calcium carbonate is a compound that results from calcium ions reacting with carbonate ions in the water. Decant the clear solution into a beaker. This reaction creates a solid particle that sinks to the ocean floor. In other words limewater is a clear colorless aqueous solution of calcium hydroxide. Add about 2 cm lime water to a test tube.
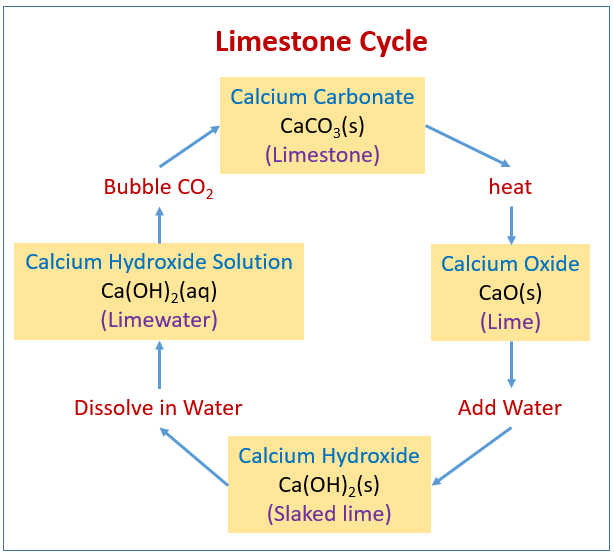 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Calcium carbonate is a compound that results from calcium ions reacting with carbonate ions in the water. Put 1 teaspoon of calcium hydroxide in a clean glass jar up to 1 gallon in size. After it is apparent that the rock has been cooked and decomposed turn the heat off. Blow through a drinking straw to bubble co 2 through the lime water solution until it goes cloudy 20 30 seconds. In other words limewater is a clear colorless aqueous solution of calcium hydroxide.
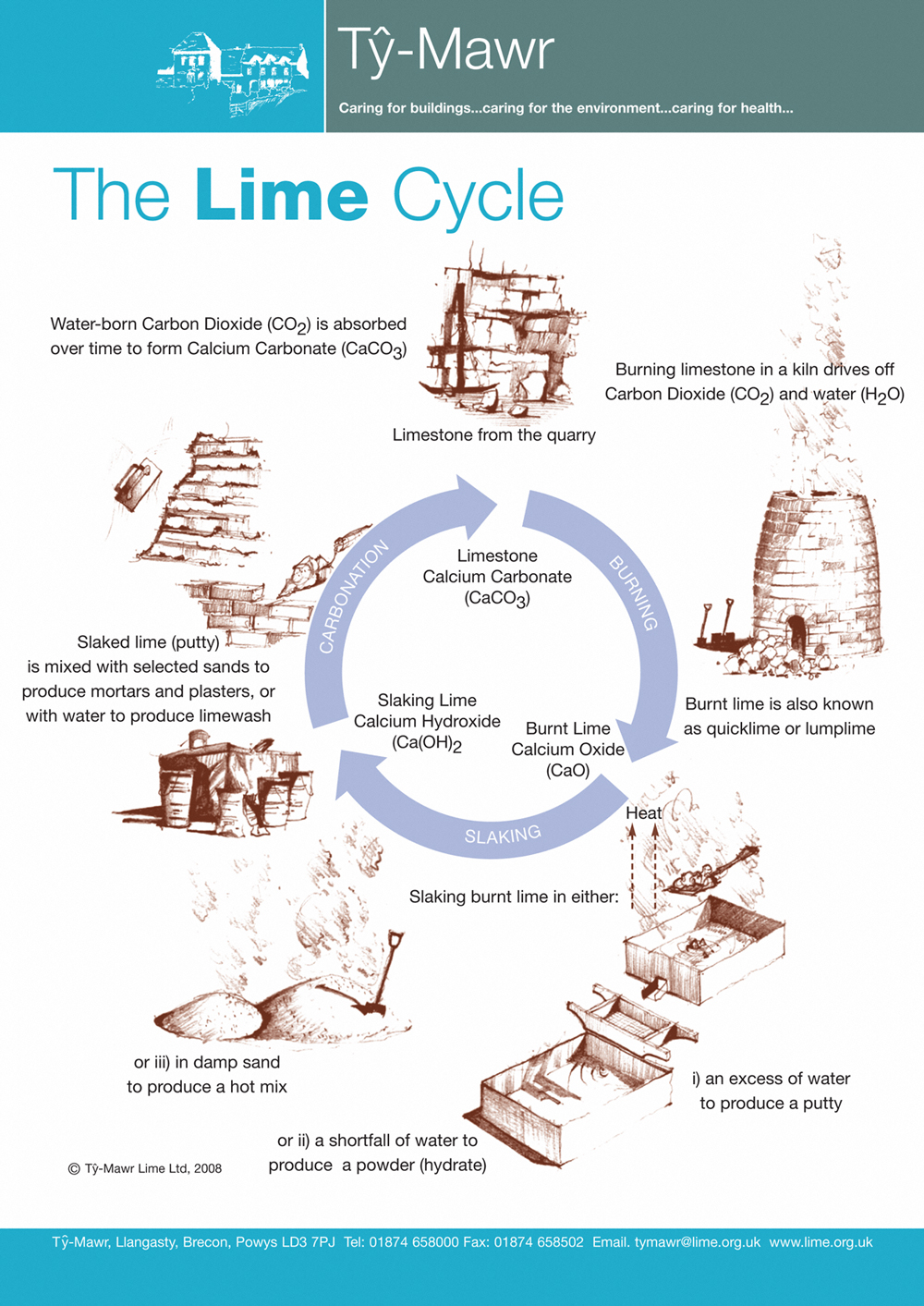 Source: lime.org.uk
Source: lime.org.uk
Put 1 teaspoon of calcium hydroxide in a clean glass jar up to 1 gallon in size. That means it is made up of calcium carbon and oxygen. Lime what is calcium carbonate. Decant the clear solution into a beaker. It is made by mixing calcium hydroxide in water 4 to 8 times the quantity of lime.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to make lime water from calcium carbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.