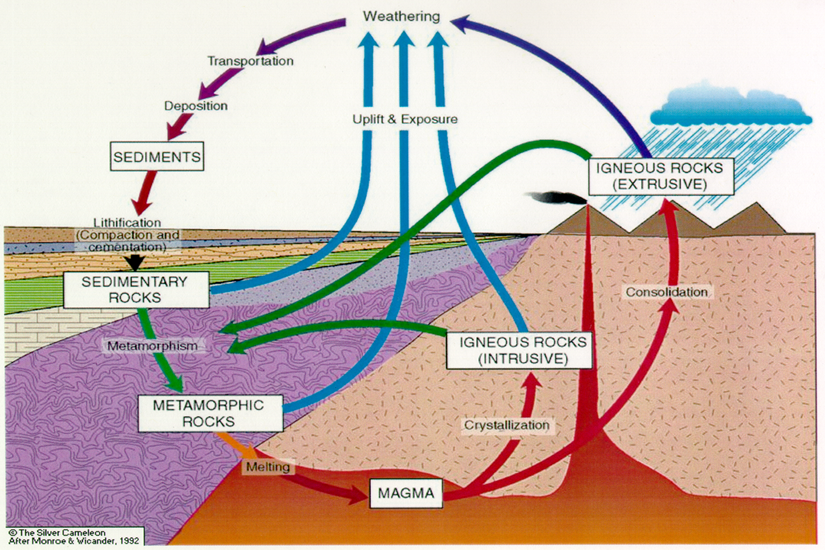
Iron nail in copper sulfate
Iron Nail In Copper Sulfate. When iron nail ferrum is dipped in copper sulphate cuso4 there takes reaction between them and coppersulphate change its color from blue to light green. Fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. Reaction of iron and copper sulphate is. 10 ml of copper sulphate solution is taken in two separate test tubes.
 Iron Nail And Copper Sulfate Youtube From youtube.com
Iron Nail And Copper Sulfate Youtube From youtube.com
Fe s cuso4 aq feso4 aq cu s in this case. This shows iron is more reactive than copper it can replace copper from cuso4. Fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. The chemical reaction can be written as. Other iron nail and test tube containing copper sulphate solution are kept for comparison. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green.
Fe cuso4 feso4 cu cuso4 is in blue color and feso4 is in light green color.
Iron nails kept in copper sulphate solution fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. This shows iron is more reactive than copper it can replace copper from cuso4. Two iron nails are taken and rubbed with a sand paper to make them shining. One of the iron nail is placed in one test tube. Iron nails kept in copper sulphate solution fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. The chemical reaction can be written as.

Fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. Reaction of iron and copper sulphate is. Iron nails kept in copper sulphate solution fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. Two iron nails are taken and rubbed with a sand paper to make them shining.
 Source: forum.byjus.com
Source: forum.byjus.com
Fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. Other iron nail and test tube containing copper sulphate solution are kept for comparison. Reaction of iron and copper sulphate is. One of the iron nail is placed in one test tube. Fe cuso4 feso4 cu cuso4 is in blue color and feso4 is in light green color.
 Source: 123rf.com
Source: 123rf.com
This shows iron is more reactive than copper it can replace copper from cuso4. 10 ml of copper sulphate solution is taken in two separate test tubes. A single displacement reaction takes place when an iron nail is placed in copper sulfate solution. Reaction of iron and copper sulphate is. One of the iron nail is placed in one test tube.

This shows iron is more reactive than copper it can replace copper from cuso4. Fe cuso4 feso4 cu cuso4 is in blue color and feso4 is in light green color. The oxidising here means a transfer of electrons the copper ions are reduced at the same time. Other iron nail and test tube containing copper sulphate solution are kept for comparison. Iron nails kept in copper sulphate solution fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green.

One of the iron nail is placed in one test tube. The oxidising here means a transfer of electrons the copper ions are reduced at the same time. Two iron nails are taken and rubbed with a sand paper to make them shining. Fe s cuso4 aq feso4 aq cu s in this case. The rust on the nail is probably a powdery coating of metallic copper which looks a rusty reddish brown the copper ions come out of solution oxidising the metallic iron atoms to make them into iron ions which dissolve.

The rust on the nail is probably a powdery coating of metallic copper which looks a rusty reddish brown the copper ions come out of solution oxidising the metallic iron atoms to make them into iron ions which dissolve. 10 ml of copper sulphate solution is taken in two separate test tubes. Other iron nail and test tube containing copper sulphate solution are kept for comparison. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. The oxidising here means a transfer of electrons the copper ions are reduced at the same time.
 Source: youtube.com
Source: youtube.com
The rust on the nail is probably a powdery coating of metallic copper which looks a rusty reddish brown the copper ions come out of solution oxidising the metallic iron atoms to make them into iron ions which dissolve. The chemical reaction can be written as. Other iron nail and test tube containing copper sulphate solution are kept for comparison. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. Iron nails kept in copper sulphate solution fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green.
 Source: alamy.com
Source: alamy.com
Fe s cuso4 aq feso4 aq cu s in this case. The oxidising here means a transfer of electrons the copper ions are reduced at the same time. Reaction of iron and copper sulphate is. Iron nails kept in copper sulphate solution fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. The rust on the nail is probably a powdery coating of metallic copper which looks a rusty reddish brown the copper ions come out of solution oxidising the metallic iron atoms to make them into iron ions which dissolve.
 Source: sciencesource.com
Source: sciencesource.com
Other iron nail and test tube containing copper sulphate solution are kept for comparison. This shows iron is more reactive than copper it can replace copper from cuso4. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. Fe s cuso4 aq feso4 aq cu s in this case. Fe cuso4 feso4 cu cuso4 is in blue color and feso4 is in light green color.

10 ml of copper sulphate solution is taken in two separate test tubes. Other iron nail and test tube containing copper sulphate solution are kept for comparison. Fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. The chemical reaction can be written as. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green.
 Source: youtube.com
Source: youtube.com
Fe cuso4 feso4 cu cuso4 is in blue color and feso4 is in light green color. The oxidising here means a transfer of electrons the copper ions are reduced at the same time. Other iron nail and test tube containing copper sulphate solution are kept for comparison. Reaction of iron and copper sulphate is. Fe s cuso4 aq cu s feso4 aq when an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green.
 Source: meritnation.com
Source: meritnation.com
When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes from blue to light green. The oxidising here means a transfer of electrons the copper ions are reduced at the same time. 10 ml of copper sulphate solution is taken in two separate test tubes. Reaction of iron and copper sulphate is. One of the iron nail is placed in one test tube.
 Source: alamy.com
Source: alamy.com
The oxidising here means a transfer of electrons the copper ions are reduced at the same time. Fe s cuso4 aq feso4 aq cu s in this case. Fe cuso4 feso4 cu cuso4 is in blue color and feso4 is in light green color. The chemical reaction can be written as. One of the iron nail is placed in one test tube.
 Source: youtube.com
Source: youtube.com
This shows iron is more reactive than copper it can replace copper from cuso4. The chemical reaction can be written as. Other iron nail and test tube containing copper sulphate solution are kept for comparison. Two iron nails are taken and rubbed with a sand paper to make them shining. The rust on the nail is probably a powdery coating of metallic copper which looks a rusty reddish brown the copper ions come out of solution oxidising the metallic iron atoms to make them into iron ions which dissolve.
 Source: alamy.com
Source: alamy.com
Fe s cuso4 aq feso4 aq cu s in this case. The chemical reaction can be written as. The oxidising here means a transfer of electrons the copper ions are reduced at the same time. One of the iron nail is placed in one test tube. Two iron nails are taken and rubbed with a sand paper to make them shining.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title iron nail in copper sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.