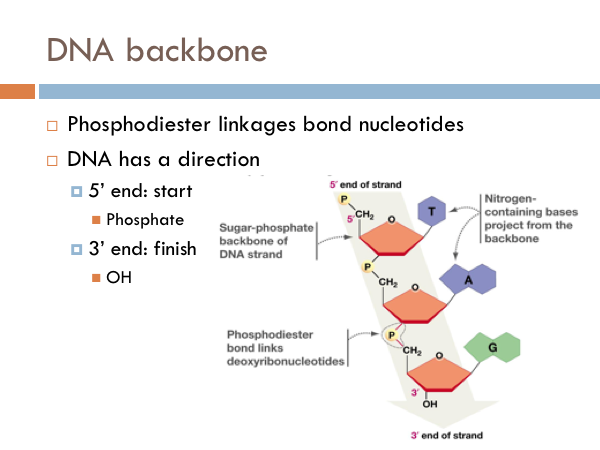
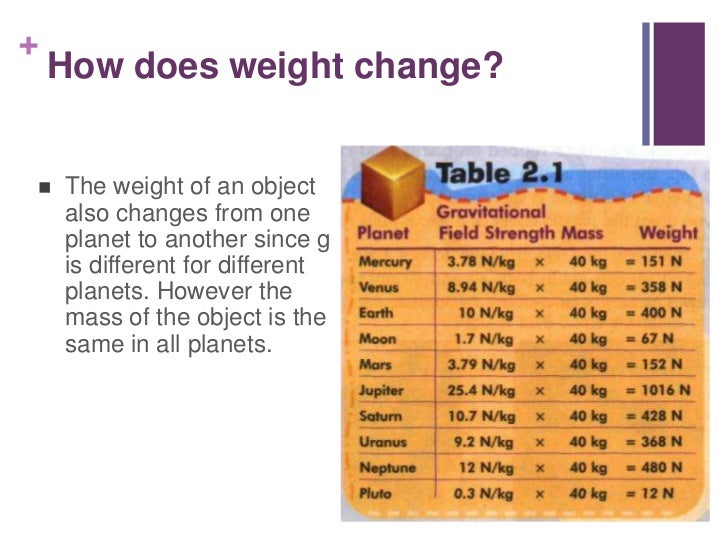
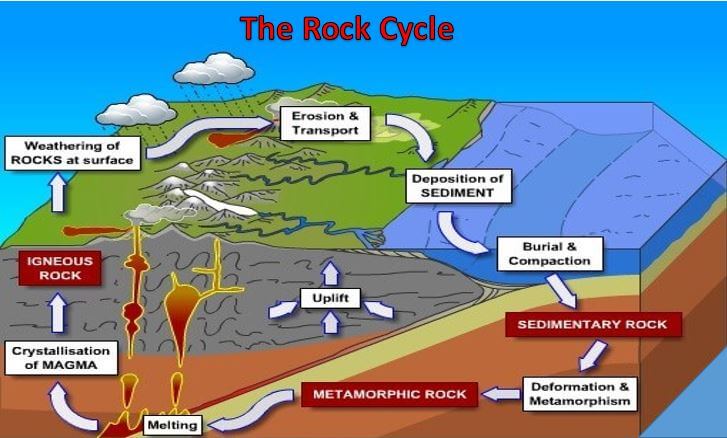
Is gold reactive or nonreactive
Is Gold Reactive Or Nonreactive. Further it is not affected by. Xe 4f 14 5d 10 6s 1 gold is also the go to element when thinking about relativistic effects. Generally speaking gold is not very reactive. Gold does not react with water.
 Learning By Questions From lbq.org
Learning By Questions From lbq.org
It combines with other gold atoms to form gold metal and it reacts chemically with other atoms. Equivalents are a measure of the actual mass of material divided by the equivalent mass. Gold does not react with water. It does not react with halogens such as chlorine or bromine very easily. This high speed 6s electron is then more attracted to the nucleus than we expect and the 6s orbital contracts relative to. Xe 4f 14 5d 10 6s 1 gold is also the go to element when thinking about relativistic effects.
Unlike silver which tarnishes when exposed to sulfur and many of its compounds gold is the only metal that does not react with sulfur.
Gold metal reacts with chlorine cl 2 or bromine br 2 to form the trihalides gold iii chloride aucl 3 or gold iii bromide aubr 3 respectively. Generally speaking gold is not very reactive. It does not react with halogens such as chlorine or bromine very easily. Gold coins for example do not corrode rust or tarnish very easily. Its 6s electron moves very quickly. The electron configuration of gold is.
 Source: quora.com
Source: quora.com
Further it is not affected by. A measure of substance equivalents that are dissolved in a volume of solution. Gold metal reacts with chlorine cl 2 or bromine br 2 to form the trihalides gold iii chloride aucl 3 or gold iii bromide aubr 3 respectively. Reaction of gold with water. Gold is generally non reactive that is why it is used in making ornaments.
 Source: metallurgyfordummies.com
Source: metallurgyfordummies.com
Gold is generally non reactive that is why it is used in making ornaments. Reaction of gold with water. The reaction for iron to rust forms iron oxide which is feasible at normal conditions but the. Gold is not very reactive but platinum is more nonreactive. It combines with other gold atoms to form gold metal and it reacts chemically with other atoms.
 Source: quora.com
Source: quora.com
A measure of substance equivalents that are dissolved in a volume of solution. Gold s 1s electron moves at around 57 7 the speed of light so the 6s electron probably quite fast. Xe 4f 14 5d 10 6s 1 gold is also the go to element when thinking about relativistic effects. A measure of substance equivalents that are dissolved in a volume of solution. The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23.

Gold coins for example do not corrode rust or tarnish very easily. A measure of substance equivalents that are dissolved in a volume of solution. It combines with other gold atoms to form gold metal and it reacts chemically with other atoms. Its 6s electron moves very quickly. Gold is not very reactive.

A measure of substance equivalents that are dissolved in a volume of solution. This high speed 6s electron is then more attracted to the nucleus than we expect and the 6s orbital contracts relative to. A measure of substance equivalents that are dissolved in a volume of solution. Reaction of gold with water. The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23.
 Source: quora.com
Source: quora.com
Reaction of gold with water. This high speed 6s electron is then more attracted to the nucleus than we expect and the 6s orbital contracts relative to. Gold as a metal is like an noble gas not very reactive. These chemical properties also account for some important uses of gold. Gold is not very reactive but platinum is more nonreactive.
 Source: newtondesk.com
Source: newtondesk.com
It does not combine with oxygen or dissolve in most acids. Gold as a metal is like an noble gas not very reactive. Gold coins for example do not corrode rust or tarnish very easily. Gold s 1s electron moves at around 57 7 the speed of light so the 6s electron probably quite fast. The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23.
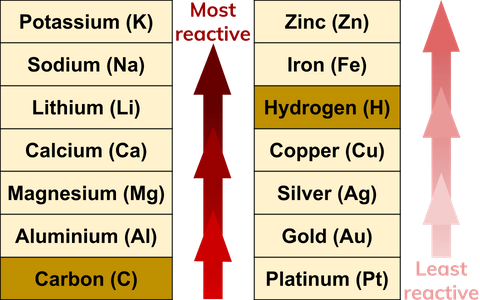 Source: senecalearning.com
Source: senecalearning.com
Its 6s electron moves very quickly. It does not combine with oxygen or dissolve in most acids. Reaction of gold with the halogens. But if you take a single gold atom it is very reactive. This high speed 6s electron is then more attracted to the nucleus than we expect and the 6s orbital contracts relative to.
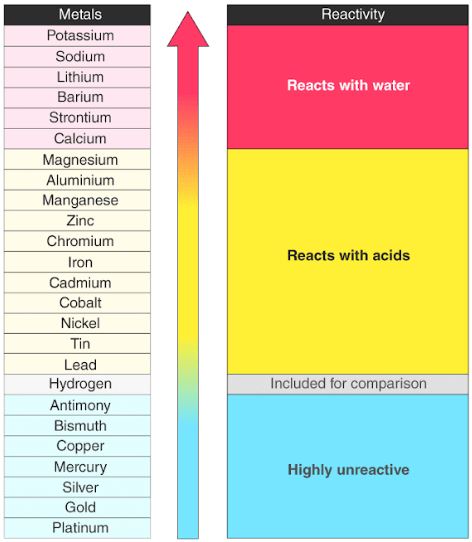 Source: byjus.com
Source: byjus.com
Non reactive elements do not easily combine with the other elements. This high speed 6s electron is then more attracted to the nucleus than we expect and the 6s orbital contracts relative to. Non reactive elements do not easily combine with the other elements. But if you take a single gold atom it is very reactive. Xe 4f 14 5d 10 6s 1 gold is also the go to element when thinking about relativistic effects.

Equivalents are a measure of the actual mass of material divided by the equivalent mass. The electron configuration of gold is. Xe 4f 14 5d 10 6s 1 gold is also the go to element when thinking about relativistic effects. Gold as a metal is like an noble gas not very reactive. The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23.
 Source: meritnation.com
Source: meritnation.com
The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23. These chemical properties also account for some important uses of gold. The reaction for iron to rust forms iron oxide which is feasible at normal conditions but the. Reaction of gold with the halogens. The electron configuration of gold is.
 Source: lbq.org
Source: lbq.org
Gold metal reacts with chlorine cl 2 or bromine br 2 to form the trihalides gold iii chloride aucl 3 or gold iii bromide aubr 3 respectively. Helium neon and argon are examples of very non reactive elements. Equivalents are a measure of the actual mass of material divided by the equivalent mass. Reaction of gold with water. Reaction of gold with the halogens.
 Source: copperalliance.org.uk
Source: copperalliance.org.uk
Reaction of gold with water. It does not react with halogens such as chlorine or bromine very easily. Unlike silver which tarnishes when exposed to sulfur and many of its compounds gold is the only metal that does not react with sulfur. The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23. Equivalents are a measure of the actual mass of material divided by the equivalent mass.
 Source: lbq.org
Source: lbq.org
Gold metal reacts with chlorine cl 2 or bromine br 2 to form the trihalides gold iii chloride aucl 3 or gold iii bromide aubr 3 respectively. Gold metal reacts with chlorine cl 2 or bromine br 2 to form the trihalides gold iii chloride aucl 3 or gold iii bromide aubr 3 respectively. It does not react with halogens such as chlorine or bromine very easily. Helium neon and argon are examples of very non reactive elements. The electronegativity of gold is 1 93 but the electronegativity of platinum is 2 23.
 Source: chemlegin.wordpress.com
Source: chemlegin.wordpress.com
Gold does not react with water. However gold does dissolve in aqueous cyanide solutions in the presence of air. Xe 4f 14 5d 10 6s 1 gold is also the go to element when thinking about relativistic effects. This high speed 6s electron is then more attracted to the nucleus than we expect and the 6s orbital contracts relative to. Non reactive elements do not easily combine with the other elements.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is gold reactive or nonreactive by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.