Polyvinyl alcohol borax
Polyvinyl Alcohol Borax. Slime polyvinyl alcohol with borax description. 100 ml of the 4 poly vinyl alcohol is added to a styrofoam cup. Polyvinyl alcohol hot plate w thermometer. Here is the procedure of how the polyvinyl slime is prepared.
 High Water Content Mouldable Polyvinyl Alcohol Borax Hydrogels Reinforced By Well Dispersed Cellulose Nanoparticles Dynamic Rheological Properties And Hydrogel Formation Mechanism Sciencedirect From sciencedirect.com
High Water Content Mouldable Polyvinyl Alcohol Borax Hydrogels Reinforced By Well Dispersed Cellulose Nanoparticles Dynamic Rheological Properties And Hydrogel Formation Mechanism Sciencedirect From sciencedirect.com
In this activity some interesting properties of the slime are investigated. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. This demonstration is typically performed as a hands on activity for younger audiences but can also be used to discuss polymerization. The effects of particle size aspect ratio crystal structure and surface charge of cnps on the rheological properties of the composite hydrogels were investigated. The hydrogel features strain larger than 5000 good water retention self healing and tunable conductivity and adhesive capabilities. In this ionic hydrogel polyvinyl alcohol and borax offer the high stretchability and conductivity respectively while silk fibroin improves the stability of the hydrogel and increases water uptake by the gels.
Polyvinyl alcohol silk fibroin borax hydrogel ionotronics.
This demonstration is typically performed as a hands on activity for younger audiences but can also be used to discuss polymerization. Acs applied materials interfaces 2019 11 26 23632 23638. The rheological measurements confirmed the. Two clear colorless liquids are mixed and immediately form a gel with interesting physical properties. Polyvinyl alcohol slime in this experiment a polymer polyvinyl alcohol chemically reacts with borax to form a crosslinked polymer network. This type of polymer composed of strands of pva whose side is linked by loosely bounded borate molecules.
 Source: researchgate.net
Source: researchgate.net
In this activity some interesting properties of the slime are investigated. Cellulose nanoparticle cnp reinforced polyvinyl alcohol borax pb hydrogels were produced via a facile approach in an aqueous system. A highly stretchable self healable and biocompatible sensing platform. Two clear colorless liquids are mixed and immediately form a gel with interesting physical properties. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w.
 Source: matse1.matse.illinois.edu
Source: matse1.matse.illinois.edu
Slime polyvinyl alcohol with borax description. The formula is often improperly written as na 2 b 4 o 7 n 2 h 2 o reflecting an older incorrect understanding of the anion s molecular structure. Here is the procedure of how the polyvinyl slime is prepared. The hydrogel features strain larger than 5000 good water retention self healing and tunable conductivity and adhesive capabilities. The rheological measurements confirmed the.
 Source: sciencedirect.com
Source: sciencedirect.com
Borax also known as sodium borate sodium tetraborate or disodium tetraborate is a compound with formula na 2 h 4 b 4 o 9 nh 2 o or more precisely na h 2 o m 2 b 4 o 5 oh 2 4. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. A solution of polyvinyl alcohol pva can be made into a slime by adding borax solution which creates cross links between polymer chains. A highly stretchable self healable and biocompatible sensing platform. The hydrogel features strain larger than 5000 good water retention self healing and tunable conductivity and adhesive capabilities.
 Source: cae.tntech.edu
Source: cae.tntech.edu
The hydrogel features strain larger than 5000 good water retention self healing and tunable conductivity and adhesive capabilities. This demonstration is typically performed as a hands on activity for younger audiences but can also be used to discuss polymerization. Polyvinyl alcohol silk fibroin borax hydrogel ionotronics. The hydrogel features strain larger than 5000 good water retention self healing and tunable conductivity and adhesive capabilities. Slime polyvinyl alcohol with borax description.
 Source: matse1.matse.illinois.edu
Source: matse1.matse.illinois.edu
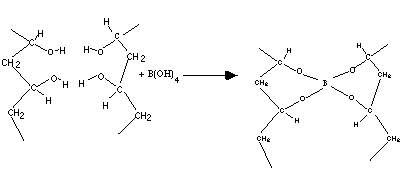
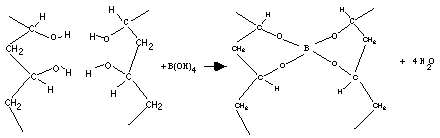
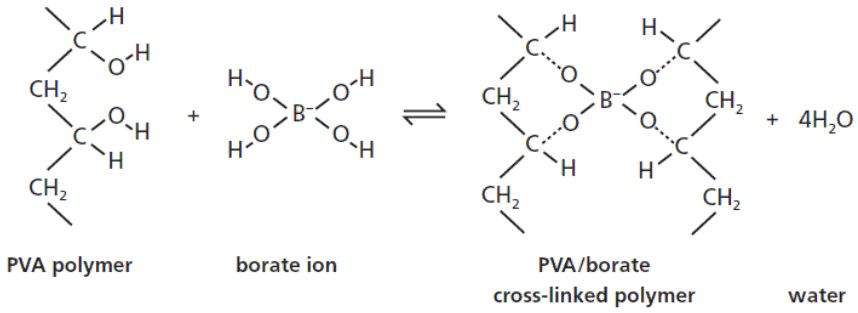
Pva when allowed to react with borax forms a crosslinked polymer network bound together by the weak hydrogen bonds. In this activity some interesting properties of the slime are investigated. Acs applied materials interfaces 2019 11 26 23632 23638. Two clear colorless liquids are mixed and immediately form a gel with interesting physical properties. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w.
 Source: edu.rsc.org
Source: edu.rsc.org
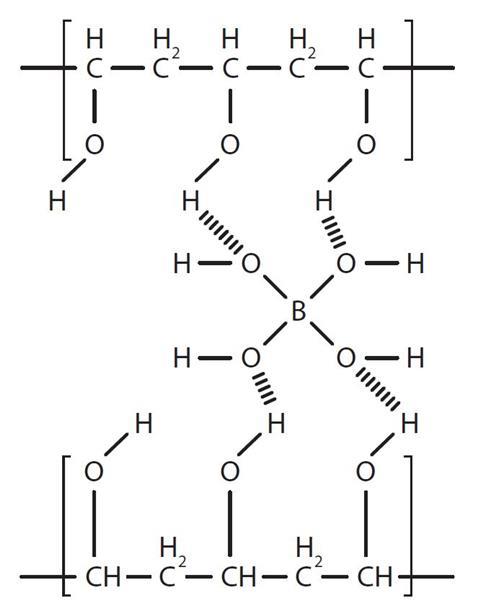
Cellulose nanoparticle cnp reinforced polyvinyl alcohol borax pb hydrogels were produced via a facile approach in an aqueous system. This type of polymer composed of strands of pva whose side is linked by loosely bounded borate molecules. Individual polymer chains are formed by covalent bonds which are strong bonds. This demonstration is typically performed as a hands on activity for younger audiences but can also be used to discuss polymerization. 100 ml of the 4 poly vinyl alcohol is added to a styrofoam cup.
Source: madsci.org
Polyvinyl alcohol silk fibroin borax hydrogel ionotronics. In making slime the individual polymer chains are bound together by weak hydrogen bonds. The rheological measurements confirmed the. In this ionic hydrogel polyvinyl alcohol and borax offer the high stretchability and conductivity respectively while silk fibroin improves the stability of the hydrogel and increases water uptake by the gels. A solution of polyvinyl alcohol pva can be made into a slime by adding borax solution which creates cross links between polymer chains.
 Source: researchgate.net
Source: researchgate.net
Pva when allowed to react with borax forms a crosslinked polymer network bound together by the weak hydrogen bonds. In this activity some interesting properties of the slime are investigated. 146 000 186 000 and ethanol 96 etoh technical were purchased from acros organics di sodium tetraborate decahydrate borax acs iso reagent titriplex iii for analysis edta ethylenediaminetetraacetic acid disodium salt dihydrate and methylene blue were supplied by merck. Polyvinyl alcohol slime in this experiment a polymer polyvinyl alcohol chemically reacts with borax to form a crosslinked polymer network. Cellulose nanoparticle cnp reinforced polyvinyl alcohol borax pb hydrogels were produced via a facile approach in an aqueous system.
 Source: edu.rsc.org
Source: edu.rsc.org
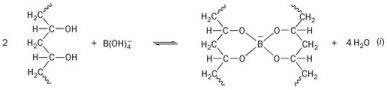
Pva when allowed to react with borax forms a crosslinked polymer network bound together by the weak hydrogen bonds. A solution of polyvinyl alcohol pva can be made into a slime by adding borax solution which creates cross links between polymer chains. Polyvinyl alcohol hot plate w thermometer. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. In this activity some interesting properties of the slime are investigated.
 Source: carolina.com
Source: carolina.com
Polyvinyl alcohol silk fibroin borax hydrogel ionotronics. Polyvinyl alcohol slime in this experiment a polymer polyvinyl alcohol chemically reacts with borax to form a crosslinked polymer network. Individual polymer chains are formed by covalent bonds which are strong bonds. Polyvinyl alcohol silk fibroin borax hydrogel ionotronics. Borax also known as sodium borate sodium tetraborate or disodium tetraborate is a compound with formula na 2 h 4 b 4 o 9 nh 2 o or more precisely na h 2 o m 2 b 4 o 5 oh 2 4.
 Source: cae.tntech.edu
Source: cae.tntech.edu
A solution of polyvinyl alcohol pva can be made into a slime by adding borax solution which creates cross links between polymer chains. This demonstration is typically performed as a hands on activity for younger audiences but can also be used to discuss polymerization. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. Simple food coloring is recommended. Individual polymer chains are formed by covalent bonds which are strong bonds.
 Source: researchgate.net
Source: researchgate.net
Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. Slime polyvinyl alcohol with borax description. Food coloring can be added to the pva in the cups to make different colors. 146 000 186 000 and ethanol 96 etoh technical were purchased from acros organics di sodium tetraborate decahydrate borax acs iso reagent titriplex iii for analysis edta ethylenediaminetetraacetic acid disodium salt dihydrate and methylene blue were supplied by merck. In this ionic hydrogel polyvinyl alcohol and borax offer the high stretchability and conductivity respectively while silk fibroin improves the stability of the hydrogel and increases water uptake by the gels.
 Source: sciencedirect.com
Source: sciencedirect.com
Two clear colorless liquids are mixed and immediately form a gel with interesting physical properties. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. The formula is often improperly written as na 2 b 4 o 7 n 2 h 2 o reflecting an older incorrect understanding of the anion s molecular structure. The polyvinyl alcohol and sodium borate are mixed together in approximately a 10 to 1 ratio. Individual polymer chains are formed by covalent bonds which are strong bonds.
 Source: researchgate.net
Source: researchgate.net
A highly stretchable self healable and biocompatible sensing platform. Acs applied materials interfaces 2019 11 26 23632 23638. Cellulose nanoparticle cnp reinforced polyvinyl alcohol borax pb hydrogels were produced via a facile approach in an aqueous system. In this activity some interesting properties of the slime are investigated. In making slime the individual polymer chains are bound together by weak hydrogen bonds.
 Source: en.m.wikipedia.org
Source: en.m.wikipedia.org
Slime polyvinyl alcohol with borax description. Individual polymer chains are formed by covalent bonds which are strong bonds. The polyvinyl alcohol and sodium borate are mixed together in approximately a 10 to 1 ratio. Polyvinyl alcohol pva 98 0 98 8 hydrolyzed m w. The rheological measurements confirmed the.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title polyvinyl alcohol borax by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





