Properties of elements compounds and mixtures
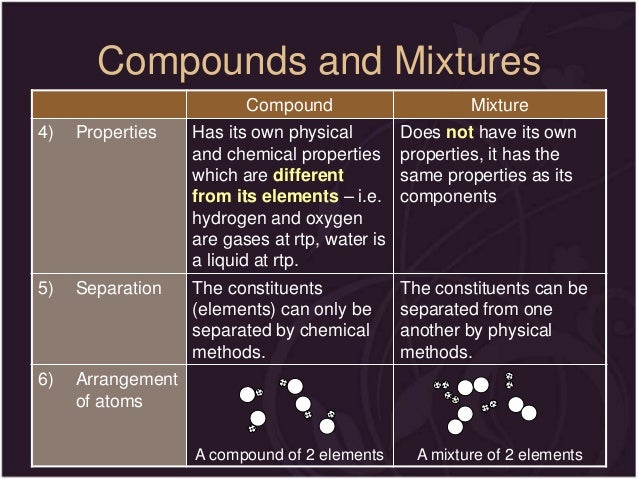
Properties Of Elements Compounds And Mixtures. Often separating the components of a homogeneous mixture is more challenging than separating the components of a heterogeneous mixture. The properties of the mixture depending upon the individual components. Elements cannot be separated into other substances. The properties of compounds are unique to themselves and need not necessarily reflect the properties of the constituent elements.
 Elements Compounds And Mixtures From slideshare.net
Elements Compounds And Mixtures From slideshare.net
The properties of compounds are unique to themselves and need not necessarily reflect the properties of the constituent elements. Pin on science elements compounds mixtures 2 1 the lightest element is. Chemical symbols and formulae are used to represent elements and compounds. Elements can be divided into metals and non metals. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Compounds can be chemically separated into their component elements.
Elements are pure substances so each element only contains one type of particle.
A pure substance is a substance in which there is only one type of particle. Often separating the components of a homogeneous mixture is more challenging than separating the components of a heterogeneous mixture. Pin on science elements compounds mixtures 2 1 the lightest element is. A homogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components cannot be visually distinguished. Boiling point and the melting point of the mixture depends upon the characteristic of the constituents. Elements can be divided into metals and non metals.
 Source: slideplayer.com
Source: slideplayer.com
Consists of atoms of two or more different elements bound together can be broken down into a simpler type of matter elements by chemical means but not by physical means has properties that are different from its component elements and. Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged. Elements can be divided into metals and non metals. The constituents of a mixture do not lose their properties and so the properties of a mixture are generally the sum of the properties of its constituents. Like elements compounds have properties that allow us to identify them.
 Source: aplustopper.com
Source: aplustopper.com
Mixtures can be physically separated into their component substances. The properties of the mixture depending upon the individual components. Boiling point and the melting point of the mixture depends upon the characteristic of the constituents. The properties of compounds are unique to themselves and need not necessarily reflect the properties of the constituent elements. Pin on science elements compounds mixtures 2 1 the lightest element is.
 Source: slideshare.net
Source: slideshare.net
Mixtures can be physically separated into their component substances. Learn about physical properties like color odor melting. Like elements compounds have properties that allow us to identify them. Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged. A homogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components cannot be visually distinguished.
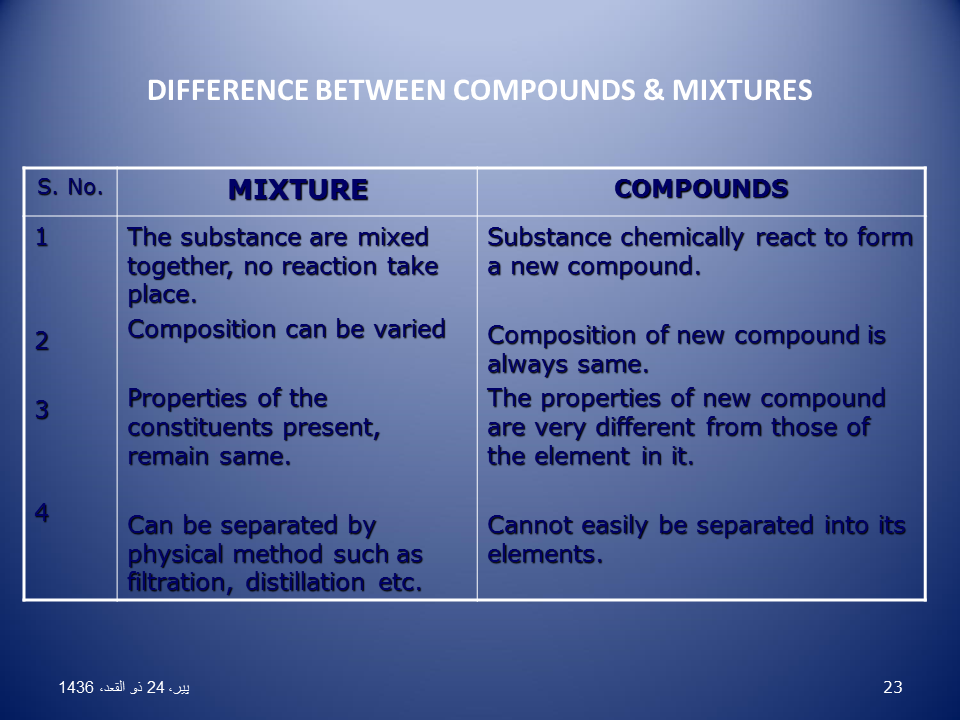
Like elements compounds have properties that allow us to identify them. Mixtures have variable properties. Mixtures can be physically separated into their component substances. Often separating the components of a homogeneous mixture is more challenging than separating the components of a heterogeneous mixture. During the formation of a mixture there is no change in energy.
 Source: youtube.com
Source: youtube.com
Compounds are made up of elements that are chemically joined. Elements are pure substances so each element only contains one type of particle. A pure substance is a substance in which there is only one type of particle. Elements and compounds are both pure substances they have the same kinds of particles throughout whilst mixtures always have more than one kind of particle. Elements can be divided into metals and non metals.
 Source: youtube.com
Source: youtube.com
Elements can be divided into metals and non metals. Mixtures can be physically separated into their component substances. This topic is school chemistry pre gcse. Choose from 500 different sets of grade 8 science elements compounds mixtures flashcards on quizlet. A homogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components cannot be visually distinguished.
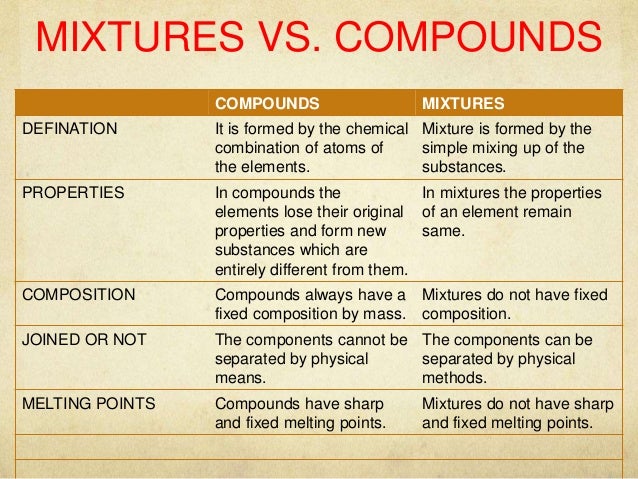 Source: slideshare.net
Source: slideshare.net
Elements and compounds are both pure substances they have the same kinds of particles throughout whilst mixtures always have more than one kind of particle. This topic is school chemistry pre gcse. Choose from 500 different sets of grade 8 science elements compounds mixtures flashcards on quizlet. A pure substance is a substance in which there is only one type of particle. Compounds can be chemically separated into their component elements.
 Source: gdmce.wordpress.com
Source: gdmce.wordpress.com
Mixtures can be physically separated into their component substances. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Elements cannot be separated into other substances. During the formation of a mixture there is no change in energy. The properties of the mixture depending upon the individual components.
 Source: pinterest.com
Source: pinterest.com
Boiling point and the melting point of the mixture depends upon the characteristic of the constituents. Compounds are made up of elements that are chemically joined. Consists of atoms of two or more different elements bound together can be broken down into a simpler type of matter elements by chemical means but not by physical means has properties that are different from its component elements and. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Chemical symbols and formulae are used to represent elements and compounds.
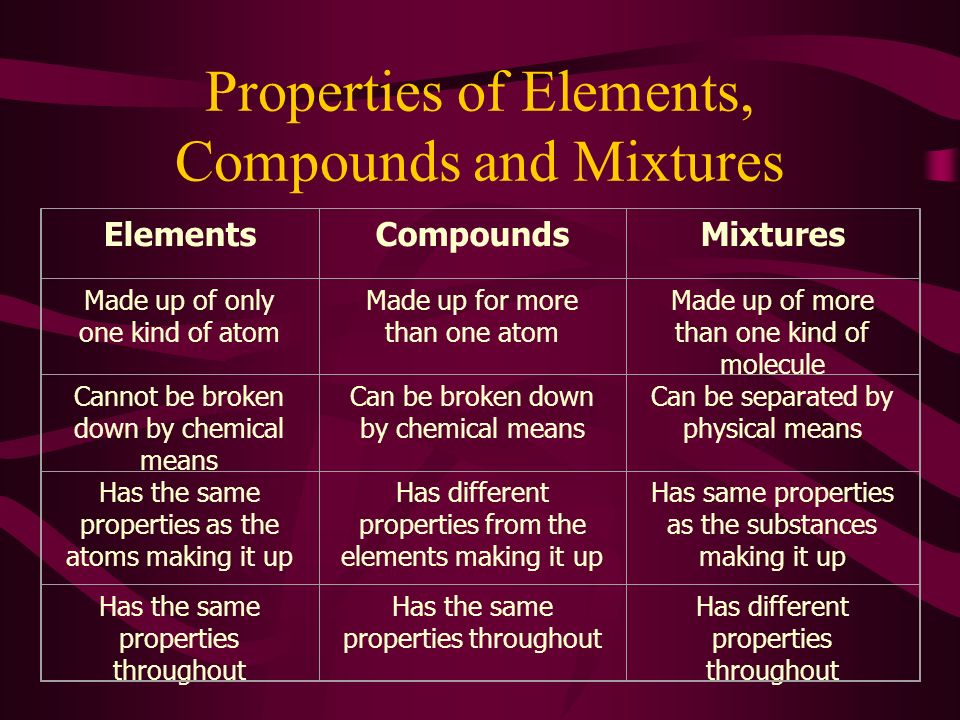 Source: slideplayer.com
Source: slideplayer.com
A pure substance is a substance in which there is only one type of particle. Elements and compounds are both pure substances they have the same kinds of particles throughout whilst mixtures always have more than one kind of particle. Consists of atoms of two or more different elements bound together can be broken down into a simpler type of matter elements by chemical means but not by physical means has properties that are different from its component elements and. The properties of compounds are unique to themselves and need not necessarily reflect the properties of the constituent elements. Elements compounds and mixtures 1.
 Source: youtube.com
Source: youtube.com
Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3. Pure substances elements and compounds have fixed properties. Chemical symbols and formulae are used to represent elements and compounds. Compounds can be chemically separated into their component elements. Boiling point and the melting point of the mixture depends upon the characteristic of the constituents.
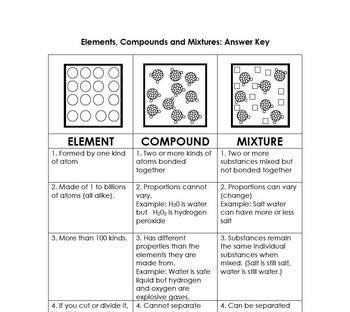 Source: teacherspayteachers.com
Source: teacherspayteachers.com
The constituents of the mixture can be separated by physical methods. Chemistry describes the structure and behaviours of different types of substances and in order to do so chemists classify different types of materials according to the particles that form them and how those particles are arranged. Learn about physical properties like color odor melting. Pure substances elements and compounds have fixed properties. Elements an element is a pure substance that cannot be separated into simpler substances by physical or chemical means 3.
 Source: learnpick.in
Source: learnpick.in
Compounds can be chemically separated into their component elements. Pin on science elements compounds mixtures 2 1 the lightest element is. A pure substance is a substance in which there is only one type of particle. Learn about physical properties like color odor melting. Elements and compounds are both pure substances they have the same kinds of particles throughout whilst mixtures always have more than one kind of particle.
 Source: learnpick.in
Source: learnpick.in
Pure substances elements and compounds have fixed properties. A homogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components cannot be visually distinguished. Often separating the components of a homogeneous mixture is more challenging than separating the components of a heterogeneous mixture. Elements can be divided into metals and non metals. Mixtures can be physically separated into their component substances.
 Source: slideshare.net
Source: slideshare.net
The properties of the mixture depending upon the individual components. Like elements compounds have properties that allow us to identify them. Always contains the same ratio of its component atoms. Elements mixtures and compounds are the names of types of chemicals. Pin on science elements compounds mixtures 2 1 the lightest element is.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title properties of elements compounds and mixtures by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.