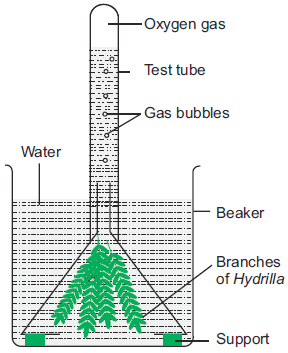
Sodium sulphate electrolysis
Sodium Sulphate Electrolysis. Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. Electrolysis of sodium sulfate solution. Cathode colorless the carbon produces purple color and there is bubble. The h ions present in the are reduced at cathode and produces h 2 gas.
 21 Electrochemistry Ppt Video Online Download From slideplayer.com
21 Electrochemistry Ppt Video Online Download From slideplayer.com
Cathode colorless the carbon produces purple color and there is bubble. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. The h ions present in the are reduced at cathode and produces h 2 gas. Electrolysis simply means passing current through a solution containing ions.
Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution.
Electrolysis of sodium sulfate solution. Cathode colorless the carbon produces purple color and there is bubble. Electrolysis of sodium sulfate solution. Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Electrolysis simply means passing current through a solution containing ions.
 Source: nurhafizahzaidipijaja.wordpress.com
Source: nurhafizahzaidipijaja.wordpress.com
When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Electrolysis simply means passing current through a solution containing ions. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
 Source: docbrown.info
Source: docbrown.info
Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. The h ions present in the are reduced at cathode and produces h 2 gas. Cathode colorless the carbon produces purple color and there is bubble. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
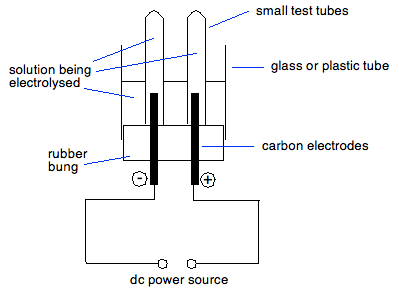 Source: chemguide.co.uk
Source: chemguide.co.uk
When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Electrolysis of sodium sulfate solution. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Cathode colorless the carbon produces purple color and there is bubble.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. Electrolysis simply means passing current through a solution containing ions. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Cathode colorless the carbon produces purple color and there is bubble. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively.
 Source: secondaryscience4all.wordpress.com
Source: secondaryscience4all.wordpress.com
Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. Cathode colorless the carbon produces purple color and there is bubble. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. Electrolysis of sodium sulfate solution.
 Source: markedbyteachers.com
Source: markedbyteachers.com
When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Electrolysis simply means passing current through a solution containing ions. Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. The h ions present in the are reduced at cathode and produces h 2 gas.
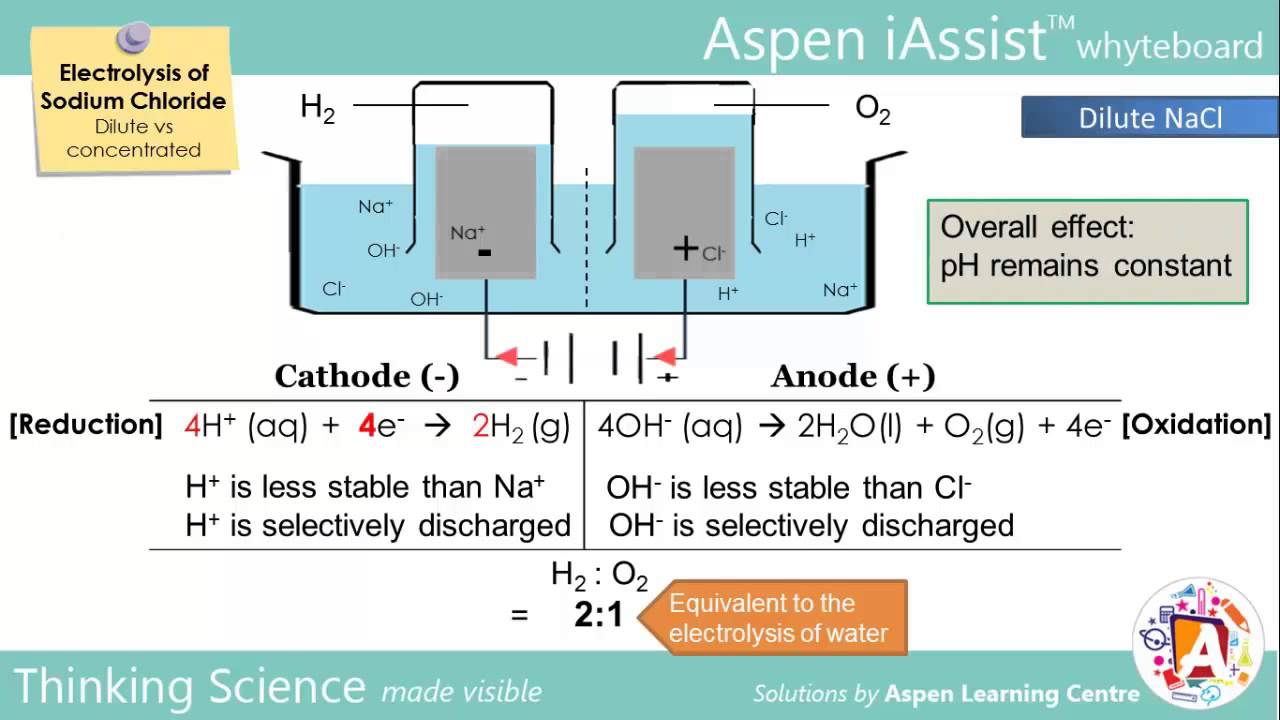 Source: m.youtube.com
Source: m.youtube.com
Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. Electrolysis simply means passing current through a solution containing ions. Electrolysis of sodium sulfate solution. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. Cathode colorless the carbon produces purple color and there is bubble.
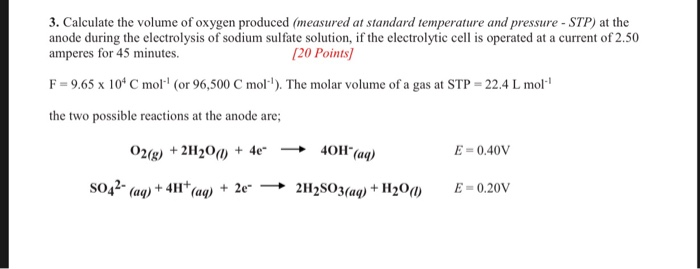 Source: chegg.com
Source: chegg.com
Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. Electrolysis simply means passing current through a solution containing ions. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Cathode colorless the carbon produces purple color and there is bubble.
 Source: youtube.com
Source: youtube.com
The h ions present in the are reduced at cathode and produces h 2 gas. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Cathode colorless the carbon produces purple color and there is bubble. Electrolysis of sodium sulfate solution.

The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Cathode colorless the carbon produces purple color and there is bubble. Electrolysis of sodium sulfate solution. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Electrolysis simply means passing current through a solution containing ions.
 Source: study.com
Source: study.com
The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Electrolysis of sodium sulfate solution. The h ions present in the are reduced at cathode and produces h 2 gas.
 Source: slideplayer.com
Source: slideplayer.com
The h ions present in the are reduced at cathode and produces h 2 gas. Cathode colorless the carbon produces purple color and there is bubble. The h ions present in the are reduced at cathode and produces h 2 gas. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water.
 Source: aplustopper.com
Source: aplustopper.com
Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. Cathode colorless the carbon produces purple color and there is bubble. Electrolysis simply means passing current through a solution containing ions. Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively.
 Source: slideplayer.com
Source: slideplayer.com
Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. Electrolysis of sodium sulfate solution. Electrolysis simply means passing current through a solution containing ions. Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
 Source: docbrown.info
Source: docbrown.info
Prepare a very concentrated it need not be saturated solution of sodium sulfate in water. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Electrolysis of sodium sulfate solution. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Add a generous portion of bromothymol blue solution to the aqueous sodium sulfate solution.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium sulphate electrolysis by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.