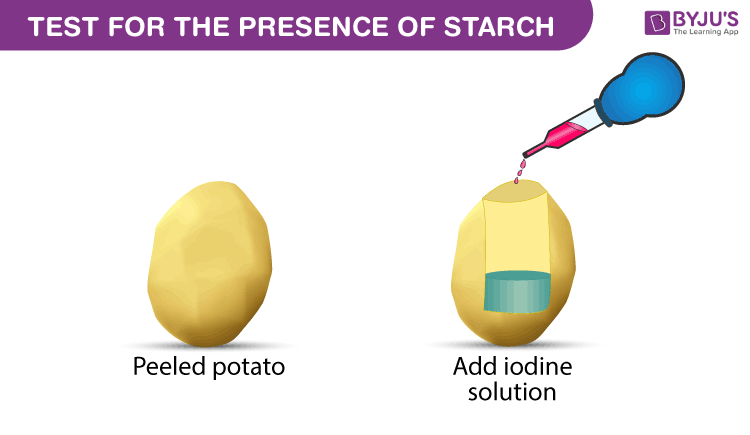
Testing starch with iodine
Testing Starch With Iodine. This test helps you to find out if a food contains starch. The deep blue color of starch iodine complexes is produced only by the free element. A blue black color results if starch is present. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves.
 Test For Starch An Overview Of Starch And Iodine Test For Starch From byjus.com
Test For Starch An Overview Of Starch And Iodine Test For Starch From byjus.com
Objectives of iodine test. The combination of starch and iodine is intensely blue black. A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. Many students who have seen the classroom demonstration where iodine crystals are gently heated in a test tube come away with the impression that liquid iodine cannot exist at atmospheric pressure. Using iodine to test for the presence of starch is a common experiment.
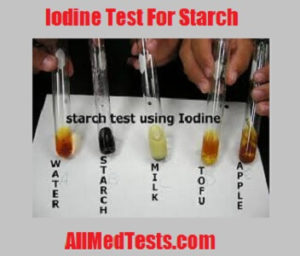
Add iodine solution to a solution or directly onto materials such as bread potato crackers.
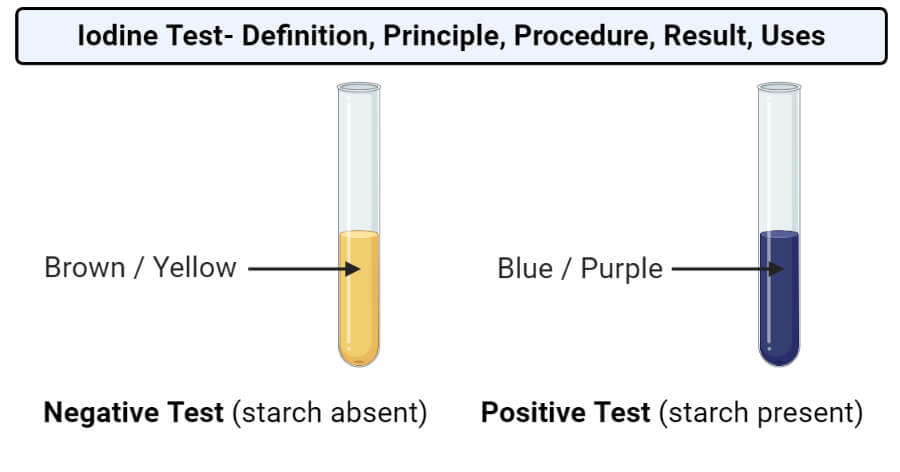
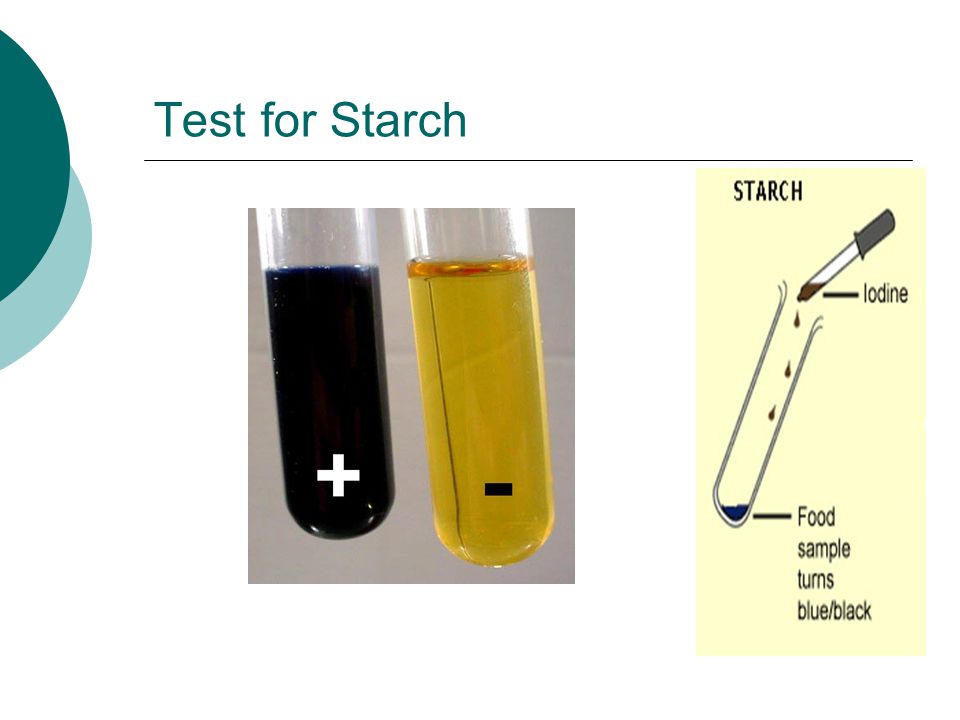
A blue black color is a positive result. Many students who have seen the classroom demonstration where iodine crystals are gently heated in a test tube come away with the impression that liquid iodine cannot exist at atmospheric pressure. The iodine test is utilized to test for the presence of starch. A blue black color results if starch is present. The brown iodine solution reacts with starch and changes it to a blue black color. Objectives of iodine test.
 Source: microbenotes.com
Source: microbenotes.com
At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. Using iodine to test for the presence of starch is a common experiment. A blue black color is a positive result. When iodine solution react with start a bluish color is obtained indicating the presence of starch but this test can be affected by temperature and ph which is explained below. Many students who have seen the classroom demonstration where iodine crystals are gently heated in a test tube come away with the impression that liquid iodine cannot exist at atmospheric pressure.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This test helps you to find out if a food contains starch. But how does this color change work. The brown iodine solution reacts with starch and changes it to a blue black color. When iodine solution react with start a bluish color is obtained indicating the presence of starch but this test can be affected by temperature and ph which is explained below. This test has a variation termed starch iodine test that is performed to indicate the presence of glucose made by plants in the leaves.
 Source: satuluqehujycozu.usagiftsshops.com
Source: satuluqehujycozu.usagiftsshops.com
If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. Using iodine to test for the presence of starch is a common experiment. The food products which we eat include different types of carbohydrates among which starch and sugars are the main carbohydrates found in our food products. A blue black color is a positive result. Iodine test is a chemical test used to distinguish mono or disaccharides from certain polysaccharides like amylase dextrin and glycogen.

Starch amylopectin does not give the color nor does cellulose nor do disaccharides such as sucrose in sugar. A blue black color results if starch is present. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. This test helps you to find out if a food contains starch. Starch is the carbohydrate storage unit of plants it can be detected in a solution via starch iodine test if you have an unknown solution and you are asked to detect whether starch is present in this solution or nor you would have to perform starch test for this test follow the procedure below.

The brown iodine solution reacts with starch and changes it to a blue black color. Starch is the carbohydrate storage unit of plants it can be detected in a solution via starch iodine test if you have an unknown solution and you are asked to detect whether starch is present in this solution or nor you would have to perform starch test for this test follow the procedure below. When iodine solution react with start a bluish color is obtained indicating the presence of starch but this test can be affected by temperature and ph which is explained below. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. Using iodine to test for the presence of starch is a common experiment.
 Source: cider.org.uk
Source: cider.org.uk
Add iodine solution to a solution or directly onto materials such as bread potato crackers. The combination of starch and iodine is intensely blue black. Starch amylopectin does not give the color nor does cellulose nor do disaccharides such as sucrose in sugar. A blue black color results if starch is present. If starch amylose is not present then the color will stay orange or yellow.
 Source: nku.edu
Source: nku.edu
If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. The food products which we eat include different types of carbohydrates among which starch and sugars are the main carbohydrates found in our food products. This test helps you to find out if a food contains starch. Iodine test is a chemical test used to distinguish mono or disaccharides from certain polysaccharides like amylase dextrin and glycogen. But how does this color change work.
 Source: sciencesource.com
Source: sciencesource.com
If starch amylose is not present then the color will stay orange or yellow. The combination of starch and iodine is intensely blue black. The brown iodine solution reacts with starch and changes it to a blue black color. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. Abstract the absorbance pattern of amylose iodine complex from cassava starch sample was examined by scanning of uv visible spectra between 200 800 nm.
 Source: igbiologyy.blogspot.com
Source: igbiologyy.blogspot.com
If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. The combination of starch and iodine is intensely blue black. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. A blue black color results if starch is present. If starch amylose is not present then the color will stay orange or yellow.
 Source: alamy.com
Source: alamy.com
The combination of starch and iodine is intensely blue black. The iodine test is utilized to test for the presence of starch. A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color. The iodine test for starch is mainly performed to test the presence of carbohydrates. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine.
 Source: socratic.org
Source: socratic.org
The interaction between starch and the triiodide anion i 3 is the basis for iodometry. At the point when treated with iki solution iodine broke up in a watery arrangement of potassium iodide the tri iodide anion edifices with starch creating a serious blue purple coloring. The interaction between starch and the triiodide anion i 3 is the basis for iodometry. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. Using iodine to test for the presence of starch is a common experiment.
 Source: allmedtests.com
Source: allmedtests.com
The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance. If starch amylose is not present then the color will stay orange or yellow. Add iodine solution to a solution or directly onto materials such as bread potato crackers. The deep blue color of starch iodine complexes is produced only by the free element. The brown iodine solution reacts with starch and changes it to a blue black color.
 Source: youtube.com
Source: youtube.com
Add iodine solution to a solution or directly onto materials such as bread potato crackers. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine. Many students who have seen the classroom demonstration where iodine crystals are gently heated in a test tube come away with the impression that liquid iodine cannot exist at atmospheric pressure. If starch amylose is not present then the color will stay orange or yellow. The cassava starch sample was analyzed at the different conditions to investigate maximum wavelength and absorbance.
 Source: byjus.com
Source: byjus.com
The brown iodine solution reacts with starch and changes it to a blue black color. A blue black color is a positive result. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. This test helps you to find out if a food contains starch. Iodine test is a chemical test used to distinguish mono or disaccharides from certain polysaccharides like amylase dextrin and glycogen.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
If starch amylose is not present then the color will stay orange or yellow. A blue black color results if starch is present. The iodine test for starch is mainly performed to test the presence of carbohydrates. This test helps you to find out if a food contains starch. The combination of starch and iodine is intensely blue black.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title testing starch with iodine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.