What determines the reactivity of a metal
What Determines The Reactivity Of A Metal. Reaction between metals and acids. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. These reactions also involve the liberation of hydrogen gas. For example magnesium metal can knock hydrogen ions out of solution so it is considered more reactive than elemental hydrogen.
 Metals Learning Objectives Use Reactivity Data To Determine A Reactivity Series Relate Extraction Method To Reactivity Of Metals Write Word Symbol Equations Ppt Download From slideplayer.com
Metals Learning Objectives Use Reactivity Data To Determine A Reactivity Series Relate Extraction Method To Reactivity Of Metals Write Word Symbol Equations Ppt Download From slideplayer.com
Explanation the number of valence electrons determines an element s reactivity or how likely the element is to react with other elements. An activity series is a list of substances ranked in order of relative reactivity. The shininess of its surface. For example magnesium metal can knock hydrogen ions out of solution so it is considered more reactive than elemental hydrogen. The number of protons it has. The reactivity of a metal is defined by which metal it is.
Lead and the metals ranking above lead on the activity series form salts when reacted with hydrochloric acid or sulphuric acid.
The shininess of its surface. Therefore the reactivity series of metals can be used to predict the reactions between metals and water. Its boiling and melting points. Which determines the reactivity of an alkali metal. If the metal is more reactive than. The number of protons it has.
 Source: kids.britannica.com
Source: kids.britannica.com
Within a type of metal difference in reactivity can occur if the surface of the metal is oxidized or has reacted in some other way. The shininess of its surface. They are the most reactive of all metals and along with the elements in group 17 the most reactive elements. Within a type of metal difference in reactivity can occur if the surface of the metal is oxidized or has reacted in some other way. Its boiling and melting pointsthe shininess of its surfacethe number of protons it hasits ability to lose electrons.
 Source: aplustopper.com
Source: aplustopper.com
Its boiling and melting points. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. Reaction between metals and acids. All the other elements in group 1 are alkali metals. The number of protons it has.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
All the other elements in group 1 are alkali metals. If the metal is more reactive than. The number of protons it has. Basically it is determined through electrode potential. For example magnesium metal can knock hydrogen ions out of solution so it is considered more reactive than elemental hydrogen.
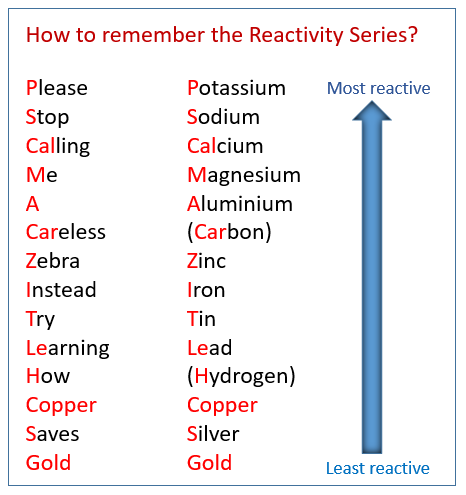 Source: onlinemathlearning.com
Source: onlinemathlearning.com
Its ability to lose electrons. Therefore the reactivity series of metals can be used to predict the reactions between metals and water. Within a type of metal difference in reactivity can occur if the surface of the metal is oxidized or has reacted in some other way. Lead and the metals ranking above lead on the activity series form salts when reacted with hydrochloric acid or sulphuric acid. They are the most reactive of all metals and along with the elements in group 17 the most reactive elements.
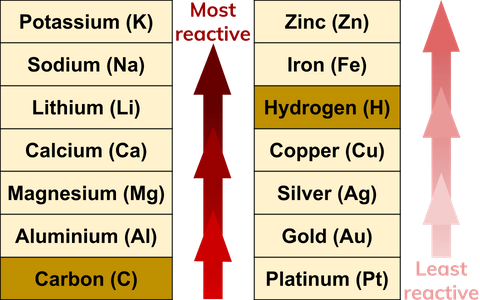 Source: senecalearning.com
Source: senecalearning.com
Explanation the number of valence electrons determines an element s reactivity or how likely the element is to react with other elements. The reactivity of a metal is determined by how tightly the metal holds onto the electrons in its outermost energy level. These reactions also involve the liberation of hydrogen gas. Its boiling and melting points. If the metal is more reactive than.
 Source: people.wou.edu
Source: people.wou.edu
Within a type of metal difference in reactivity can occur if the surface of the metal is oxidized or has reacted in some other way. Explanation the number of valence electrons determines an element s reactivity or how likely the element is to react with other elements. Lead and the metals ranking above lead on the activity series form salts when reacted with hydrochloric acid or sulphuric acid. All the other elements in group 1 are alkali metals. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting each metal with a solution of another metal ion.
 Source: socratic.org
Source: socratic.org
An activity series is a list of substances ranked in order of relative reactivity. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. The shininess of its surface. Updated february 28 2020. For example magnesium metal can knock hydrogen ions out of solution so it is considered more reactive than elemental hydrogen.
 Source: onelearningsolution.blogspot.com
Source: onelearningsolution.blogspot.com
They are the most reactive of all metals and along with the elements in group 17 the most reactive elements. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting each metal with a solution of another metal ion. All the other elements in group 1 are alkali metals. Updated february 28 2020.
 Source: slideplayer.com
Source: slideplayer.com
Reaction between metals and acids. Reaction between metals and acids. Explanation the number of valence electrons determines an element s reactivity or how likely the element is to react with other elements. Which determines the reactivity of an alkali metal. The number of protons it has.
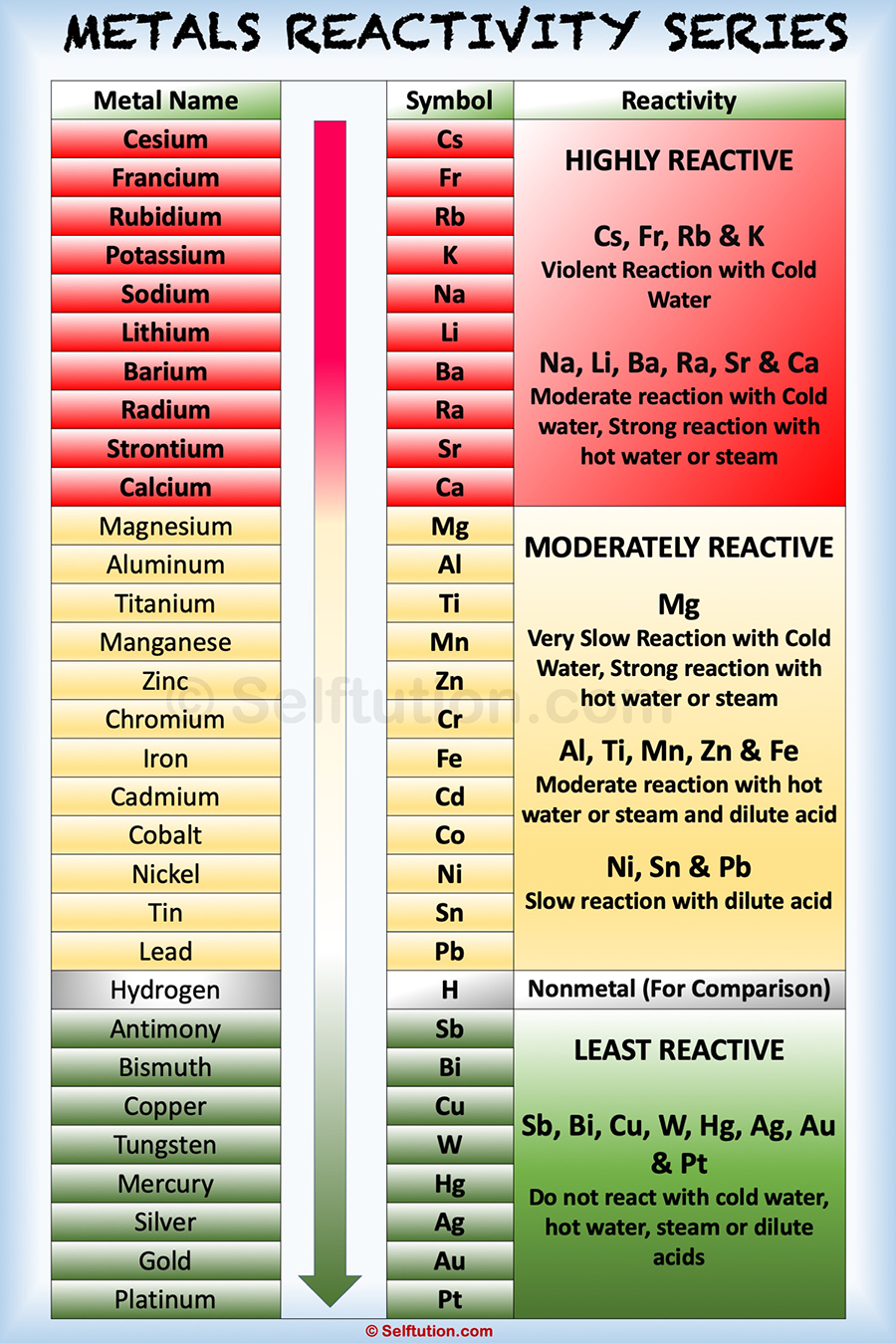 Source: selftution.com
Source: selftution.com
They are the most reactive of all metals and along with the elements in group 17 the most reactive elements. Updated february 28 2020. In order to determine the relative reactivity of each metal we will carry out displacement reactions by reacting each metal with a solution of another metal ion. Its boiling and melting pointsthe shininess of its surfacethe number of protons it hasits ability to lose electrons. If the metal is more reactive than.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The reactivity of a metal is determined by how tightly the metal holds onto the electrons in its outermost energy level. Updated february 28 2020. Basically it is determined through electrode potential. The reactivity of a metal is determined by how tightly the metal holds onto the electrons in its outermost energy level. Its boiling and melting pointsthe shininess of its surfacethe number of protons it hasits ability to lose electrons.
 Source: goodscience.com.au
Source: goodscience.com.au
The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction. Facts about solar power. Basically it is determined through electrode potential. All the other elements in group 1 are alkali metals. An activity series is a list of substances ranked in order of relative reactivity.
 Source: quora.com
Source: quora.com
Facts about solar power. Therefore the reactivity series of metals can be used to predict the reactions between metals and water. Basically it is determined through electrode potential. Its boiling and melting pointsthe shininess of its surfacethe number of protons it hasits ability to lose electrons. These electrons are called valence electrons.
Source: quora.com
Reaction between metals and acids. Its boiling and melting points. Metals usually have fewer valence electrons than nonmetals. The reactivity of a metal is determined by how tightly the metal holds onto the electrons in its outermost energy level. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction.
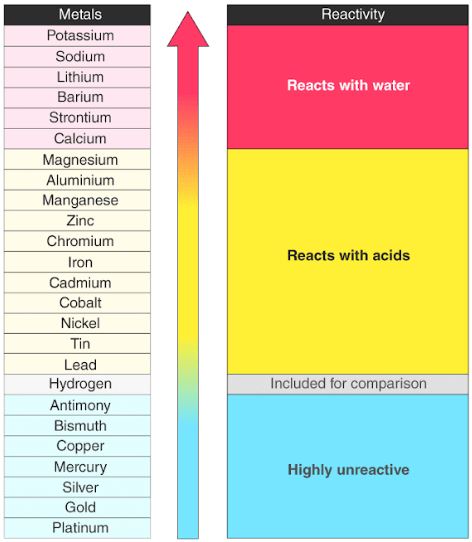 Source: byjus.com
Source: byjus.com
For example magnesium metal can knock hydrogen ions out of solution so it is considered more reactive than elemental hydrogen. Which determines the reactivity of an alkali metal. Its boiling and melting points. These electrons are called valence electrons. All the other elements in group 1 are alkali metals.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what determines the reactivity of a metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.