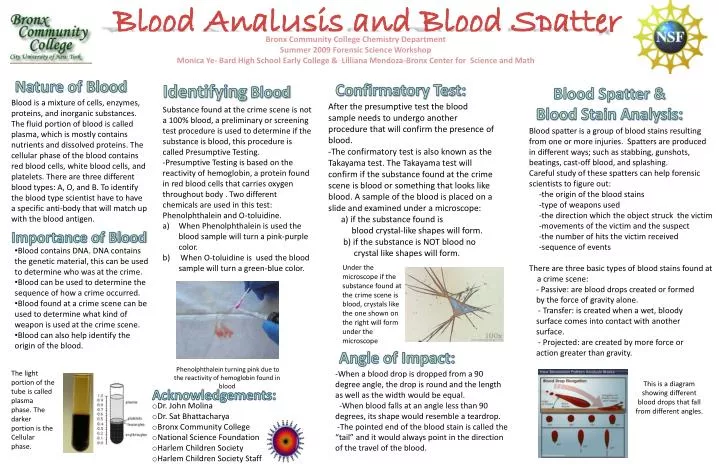
What happens when an acid reacts with a base
What Happens When An Acid Reacts With A Base. If you mix equal amounts of a strong acid and a strong base the two chemicals essentially cancel each other out and produce a salt and water. When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water. When an acid reacts with a base an acud base reaction or in other words a neutralization reaction occurs wherein salt and water is formed. All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time.
 Acid Base Reaction Wikipedia From en.wikipedia.org
Acid Base Reaction Wikipedia From en.wikipedia.org
When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph ph 7 solution. When an acid reacts with base then a salt and water is formed. If you mix equal amounts of a strong acid and a strong base the two chemicals essentially cancel each other out and produce a salt and water. All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time. Naoh aq hcl aq nacl aq h2o l such reaction is called.
Acid base salt water.
This is called a neutralization reaction and looks like this. When an acid reacts with base then a salt and water is formed. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. When an acid reacts with a base an acud base reaction or in other words a neutralization reaction occurs wherein salt and water is formed. Acid base salt water. The type if salt formed depends on the strength of the acid and base taken.
 Source: intl.siyavula.com
Source: intl.siyavula.com
If you have taken a strong base and a strong acid then you would get a neutral salt. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. If you mix equal amounts of a strong acid and a strong base the two chemicals essentially cancel each other out and produce a salt and water. The products of this reaction are a salt and water. Bases are substances that taste bitter and change the colour of red litmus paper to blue.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Bases are substances that taste bitter and change the colour of red litmus paper to blue. The reaction of an acid with a base is called a neutralization reaction. Once all the reactions are at equilibrium there is no more net change. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph ph 7 solution. When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water.
 Source: slideplayer.com
Source: slideplayer.com
All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time. Acid base salt water. The further from equilibrium a reaction is the faster is the net change. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph ph 7 solution. When an acid reacts with base then a salt and water is formed.
 Source: intl.siyavula.com
Source: intl.siyavula.com
Acid base salt water. The further from equilibrium a reaction is the faster is the net change. If you have taken a strong base and a strong acid then you would get a neutral salt. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph ph 7 solution. The type if salt formed depends on the strength of the acid and base taken.
 Source: youtube.com
Source: youtube.com
When an acid reacts with a base an acud base reaction or in other words a neutralization reaction occurs wherein salt and water is formed. When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water. When an acid reacts with base then a salt and water is formed. All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time. The further from equilibrium a reaction is the faster is the net change.
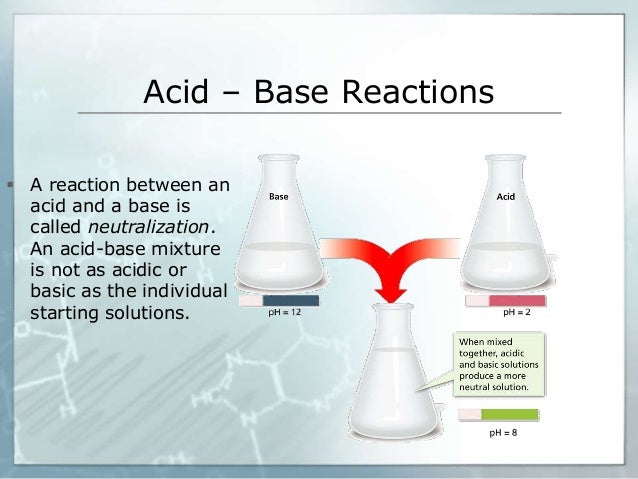 Source: slideshare.net
Source: slideshare.net
Mixing equal amounts of a strong acid with a strong base also produces a neutral ph ph 7 solution. The products of this reaction are a salt and water. The reaction of an acid with a base is called a neutralization reaction. All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time. Mixing equal amounts of a strong acid with a strong base also produces a neutral ph ph 7 solution.
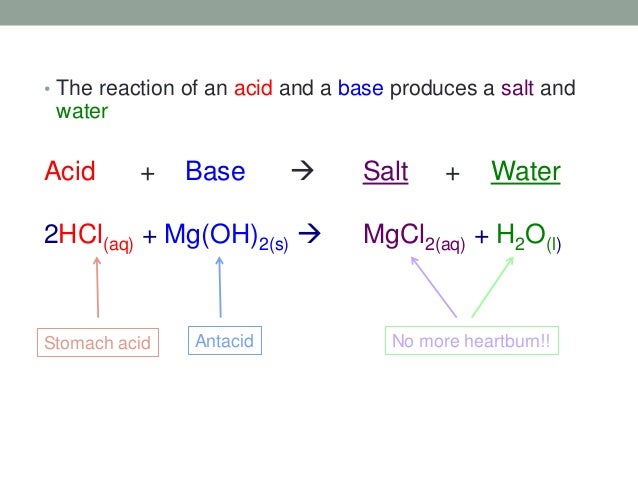 Source: slideshare.net
Source: slideshare.net
Acid base salt water. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. Naoh aq hcl aq nacl aq h2o l such reaction is called. The products of this reaction are a salt and water. The reaction of an acid with a base is called a neutralization reaction.
Source: quora.com
Once all the reactions are at equilibrium there is no more net change. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. The type if salt formed depends on the strength of the acid and base taken. The products of this reaction are a salt and water. When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water.
 Source: dronstudy.com
Source: dronstudy.com
The products of this reaction are a salt and water. The type if salt formed depends on the strength of the acid and base taken. An acid in a water solution tastes sour changes the colour of blue litmus paper to red reacts with some metals e g iron to liberate hydrogen reacts with bases to form salts and promotes certain chemical reactions acid catalysis. Bases are substances that taste bitter and change the colour of red litmus paper to blue. The reaction of an acid with a base is called a neutralization reaction.
 Source: doubtnut.com
Source: doubtnut.com
All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time. The products of this reaction are a salt and water. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. Naoh aq hcl aq nacl aq h2o l such reaction is called. When an acid reacts with base then a salt and water is formed.
 Source: youtube.com
Source: youtube.com
Once all the reactions are at equilibrium there is no more net change. Bases are substances that taste bitter and change the colour of red litmus paper to blue. This is called a neutralization reaction and looks like this. Acid base salt water. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction.
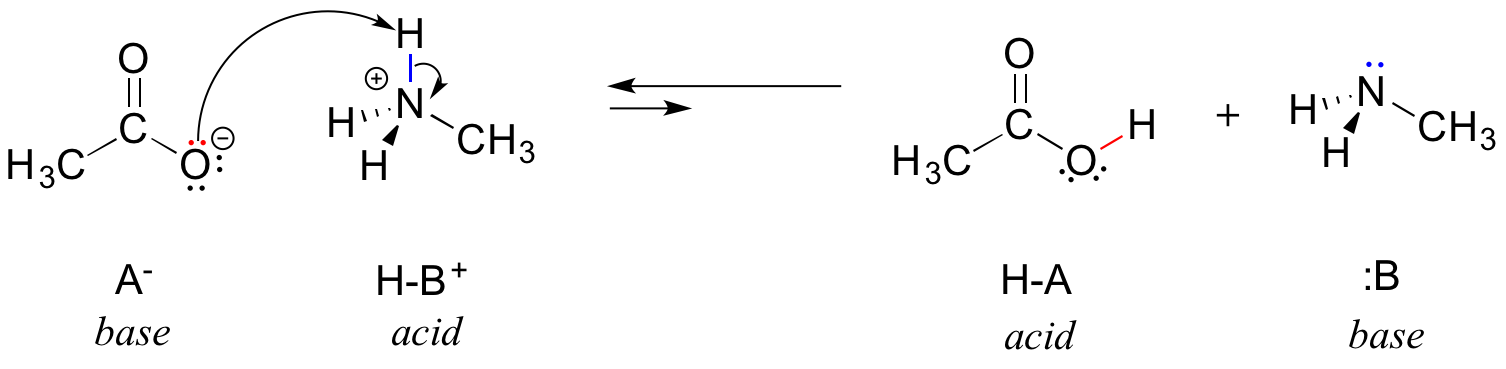 Source: chem.libretexts.org
Source: chem.libretexts.org
When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. The type if salt formed depends on the strength of the acid and base taken. An acid in a water solution tastes sour changes the colour of blue litmus paper to red reacts with some metals e g iron to liberate hydrogen reacts with bases to form salts and promotes certain chemical reactions acid catalysis. All reactions any of the acids water hydronium or ammonium reacting with any of the bases water ammonia or hydroxide go on at the same time.
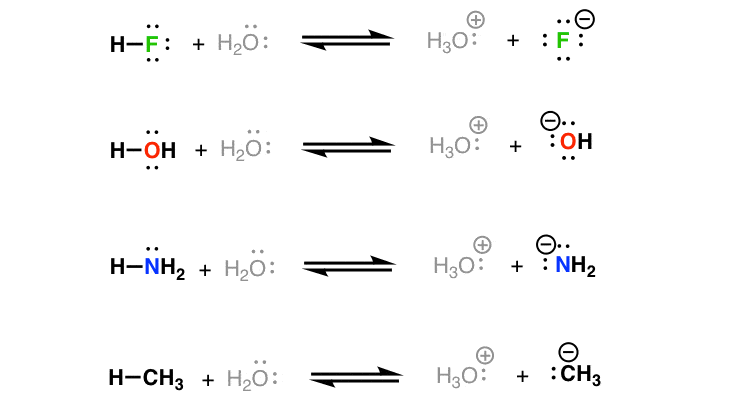 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water. The type if salt formed depends on the strength of the acid and base taken. Acid base salt water. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. When an acid reacts with base then a salt and water is formed.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Once all the reactions are at equilibrium there is no more net change. The further from equilibrium a reaction is the faster is the net change. The reaction of an acid with a base is called a neutralization reaction. The products of this reaction are a salt and water. Once all the reactions are at equilibrium there is no more net change.
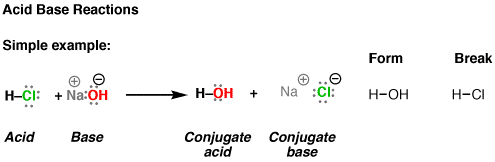 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
This is called a neutralization reaction and looks like this. The type if salt formed depends on the strength of the acid and base taken. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. When hydrochloric acid reacts with sodium hydroxide solution then a neutralisation reaction takes place to form sodium chloride and water. Once all the reactions are at equilibrium there is no more net change.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what happens when an acid reacts with a base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.