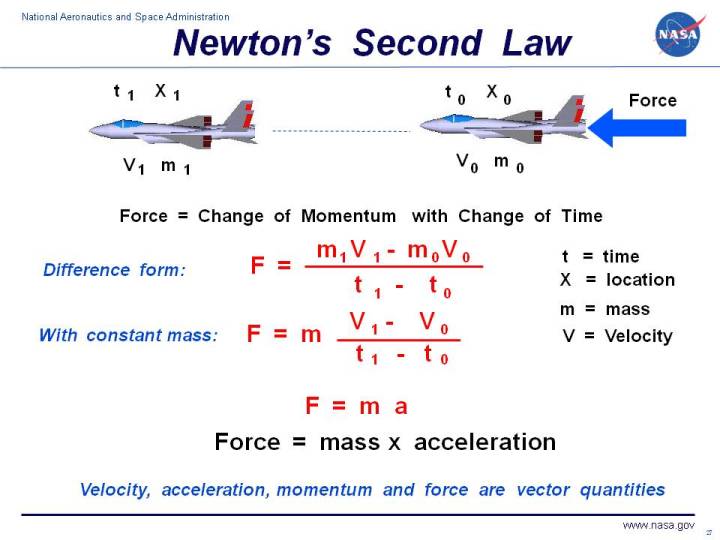
What is biuret solution
What Is Biuret Solution. The biuret test also known as piotrowski s test is a chemical test used for detecting the presence of peptide bonds. What is the biuret solution. Biuret test is the name of a chemical test which utilises the biuret reagents which contains a 1 solution of copper ii sulphate cuso. It is based on the biuret reagent a blue solution that turns violet upon contact with proteins or any substance with peptide bonds.
 Science Source Biuret Reagent From sciencesource.com
Science Source Biuret Reagent From sciencesource.com
The test and reagent do not actually contain biuret. What is the biuret solution. It is based on the biuret reagent a blue solution that turns violet upon contact with proteins or any substance with peptide bonds. The biuret reagent is made up of hydrated copper sulfate sodium hydroxide and rochelle salt sodium potassium tartrate. The biuret reagent is a solution composed of sodium hydroxide naoh or potassium hydroxide koh hydrated copper ii sulfate and potassium sodium tartrate. To confirm the presence of protein it will rely on the changes in color.
Copper salts in alkaline solution form a purple complex with substances containing two or more peptide bonds.
In the presence of peptides a copper ion forms mauve colored coordination complexes in an alkaline solution. The parent compound of the biuret group of compounds. The biuret method is a colorimetric technique specific for proteins and peptides. The biuret reagent is made up of hydrated copper sulfate sodium hydroxide and rochelle salt sodium potassium tartrate. This test is given by compounds containing two or more peptide bond co nh group. It is the cu in the biuret reagent that forms a complex with the peptide bonds found in proteins.
 Source: sciencecompany.com
Source: sciencecompany.com
The biuret test also known as piotrowski s test is a chemical test used for detecting the presence of peptide bonds. The biuret reaction can be used to assess the concentration of proteins because peptide bonds occur with the same frequency per amino acid in the peptide. A biuret test is a chemical assay that helps check for the presence of protein in a given sample. Biuret is a member of the class of condensed ureas that is the compound formed by the condensation of two molecules of urea. The biuret test also known as piotrowski s test is a chemical test used for detecting the presence of peptide bonds.
 Source: amazon.com
Source: amazon.com
Although the test is called biuret it does not use the chemical biuret. Biuret is a compound formed by heating urea to 180 c. It is the cu in the biuret reagent that forms a complex with the peptide bonds found in proteins. The biuret test is a chemical test for proteins and polypeptides. This is the basis of biuret test widely used for identification of proteins and amino acids.
 Source: amazon.com
Source: amazon.com
Biuret is a compound formed by heating urea to 180 c. This is the basis of biuret test widely used for identification of proteins and amino acids. When biuret is treated with dilute copper sulfate in alkaline condition a purple colored compound is formed. The biuret reagent is made up of hydrated copper sulfate sodium hydroxide and rochelle salt sodium potassium tartrate. This test is given by compounds containing two or more peptide bond co nh group.
 Source: fishersci.com
Source: fishersci.com
Biuret is a compound formed by heating urea to 180 c. They are so named because both biuret and proteins have the same response to the test. Copper salts in alkaline solution form a purple complex with substances containing two or more peptide bonds. It is the cu in the biuret reagent that forms a complex with the peptide bonds found in proteins. The test and reagent do not actually contain biuret.
 Source: en.wikipedia.org
Source: en.wikipedia.org
An indicator that protein is present is when the color changes to violet. A substance derived from urea. Several variants on the test have been developed such as the bca test and the modified lowry test. The biuret reagent is made up of hydrated copper sulfate sodium hydroxide and rochelle salt sodium potassium tartrate. Copper salts in alkaline solution form a purple complex with substances containing two or more peptide bonds.
 Source: sciencesource.com
Source: sciencesource.com
It is the cu in the biuret reagent that forms a complex with the peptide bonds found in proteins. Biuret is a member of the class of condensed ureas that is the compound formed by the condensation of two molecules of urea. The biuret reagent is a solution composed of sodium hydroxide naoh or potassium hydroxide koh hydrated copper ii sulfate and potassium sodium tartrate. Here the rochelle salt acts as a chelating agent and stabilizes the copper ii ions. Biuret test is the name of a chemical test which utilises the biuret reagents which contains a 1 solution of copper ii sulphate cuso.
 Source: laboratoryinfo.com
Source: laboratoryinfo.com
It is based on the biuret reagent a blue solution that turns violet upon contact with proteins or any substance with peptide bonds. Although the test is called biuret it does not use the chemical biuret. To confirm the presence of protein it will rely on the changes in color. They are so named because both biuret and proteins have the same response to the test. The biuret solution used for detecting peptide bonds in made up of sodium hydroxide naoh copper ii sulfate cuso4 and sodium potassium tartrate.
 Source: onlinebiologynotes.com
Source: onlinebiologynotes.com
It is based on the biuret reagent a blue solution that turns violet upon contact with proteins or any substance with peptide bonds. A biuret test is a chemical assay that helps check for the presence of protein in a given sample. To confirm the presence of protein it will rely on the changes in color. The biuret reagent is made up of hydrated copper sulfate sodium hydroxide and rochelle salt sodium potassium tartrate. This is the basis of biuret test widely used for identification of proteins and amino acids.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
They are so named because both biuret and proteins have the same response to the test. Copper salts in alkaline solution form a purple complex with substances containing two or more peptide bonds. The biuret test also known as piotrowski s test is a chemical test used for detecting the presence of peptide bonds. In the presence of peptides a copper ion forms mauve colored coordination complexes in an alkaline solution. The test and reagent do not actually contain biuret.
 Source: sciencephoto.com
Source: sciencephoto.com
Here the rochelle salt acts as a chelating agent and stabilizes the copper ii ions. Biuret is a member of the class of condensed ureas that is the compound formed by the condensation of two molecules of urea. The biuret solution used for detecting peptide bonds in made up of sodium hydroxide naoh copper ii sulfate cuso4 and sodium potassium tartrate. They are so named because both biuret and proteins have the same response to the test. Biuret is a compound produced by heating urea at 180.
 Source: study.com
Source: study.com
What is the biuret solution. Here the rochelle salt acts as a chelating agent and stabilizes the copper ii ions. Although the test is called biuret it does not use the chemical biuret. The biuret solution used for detecting peptide bonds in made up of sodium hydroxide naoh copper ii sulfate cuso4 and sodium potassium tartrate. This test is given by compounds containing two or more peptide bond co nh group.
 Source: chemistrylearner.com
Source: chemistrylearner.com
To confirm the presence of protein it will rely on the changes in color. What is the biuret solution. An indicator that protein is present is when the color changes to violet. The biuret solution used for detecting peptide bonds in made up of sodium hydroxide naoh copper ii sulfate cuso4 and sodium potassium tartrate. Biuret is a compound formed by heating urea to 180 c.
 Source: onlinesciencenotes.com
Source: onlinesciencenotes.com
Used as a non protein nitrogen source in ruminant feed. The biuret reagent is a solution composed of sodium hydroxide naoh or potassium hydroxide koh hydrated copper ii sulfate and potassium sodium tartrate. The biuret method is a colorimetric technique specific for proteins and peptides. The biuret reagent is made up of hydrated copper sulfate sodium hydroxide and rochelle salt sodium potassium tartrate. When biuret is treated with dilute copper sulfate in alkaline condition a purple colored compound is formed.
 Source: homesciencetools.com
Source: homesciencetools.com
The biuret reagent is a solution composed of sodium hydroxide naoh or potassium hydroxide koh hydrated copper ii sulfate and potassium sodium tartrate. The test and reagent do not actually contain biuret. The biuret test also known as piotrowski s test is a chemical test used for detecting the presence of peptide bonds. It is the cu in the biuret reagent that forms a complex with the peptide bonds found in proteins. This test is given by compounds containing two or more peptide bond co nh group.
 Source: hypermol.com
Source: hypermol.com
To confirm the presence of protein it will rely on the changes in color. The biuret reagent is a solution composed of sodium hydroxide naoh or potassium hydroxide koh hydrated copper ii sulfate and potassium sodium tartrate. To confirm the presence of protein it will rely on the changes in color. When biuret is treated with dilute copper sulfate in alkaline condition a purple colored compound is formed. Biuret is a member of the class of condensed ureas that is the compound formed by the condensation of two molecules of urea.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is biuret solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.