Why is ice lighter than water
Why Is Ice Lighter Than Water. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. Solid water or ice is less dense than liquid water. When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. Water expands when it freezes making it less dense the lower the density the lighter it is.
 Why Water Is More Dense Than Ice From thoughtco.com
Why Water Is More Dense Than Ice From thoughtco.com
By staff writer last updated apr 2 2020 11 56 39 am et. These bondings leave behind the larger amount of empty space due to which density decreases. Water is unusual in that its maximum density occurs as a liquid rather than as a solid. Density is the mass per unit volume of a material. As a result the molecules spread out and so ice can float in water. The additional space created reduces the density of the water as it freezes making ice less dense than water.
The question then becomes.
Something must be happening to. For all substances density changes with temperature the mass of material does not change but the volume or space that it occupies either increases or decreases with temperature. As a result the molecules spread out and so ice can float in water. Density is the mass per unit volume of a material. This means ice floats on water. Solid water or ice is less dense than liquid water.
Source: quora.com
The density of water is 1 gm cm 3. The ice is lighter than the water because the density of ice is lesser than that of water. As a result the molecules spread out and so ice can float in water. Ice is less dense than water. Something must be happening to.
Source: quora.com
Water is unusual in that its maximum density occurs as a liquid rather than as a solid. Water expands when it freezes making it less dense the lower the density the lighter it is. By staff writer last updated apr 2 2020 11 56 39 am et. Why is ice which is water in solid form lighter than water in its liquid form. This means ice floats on water.
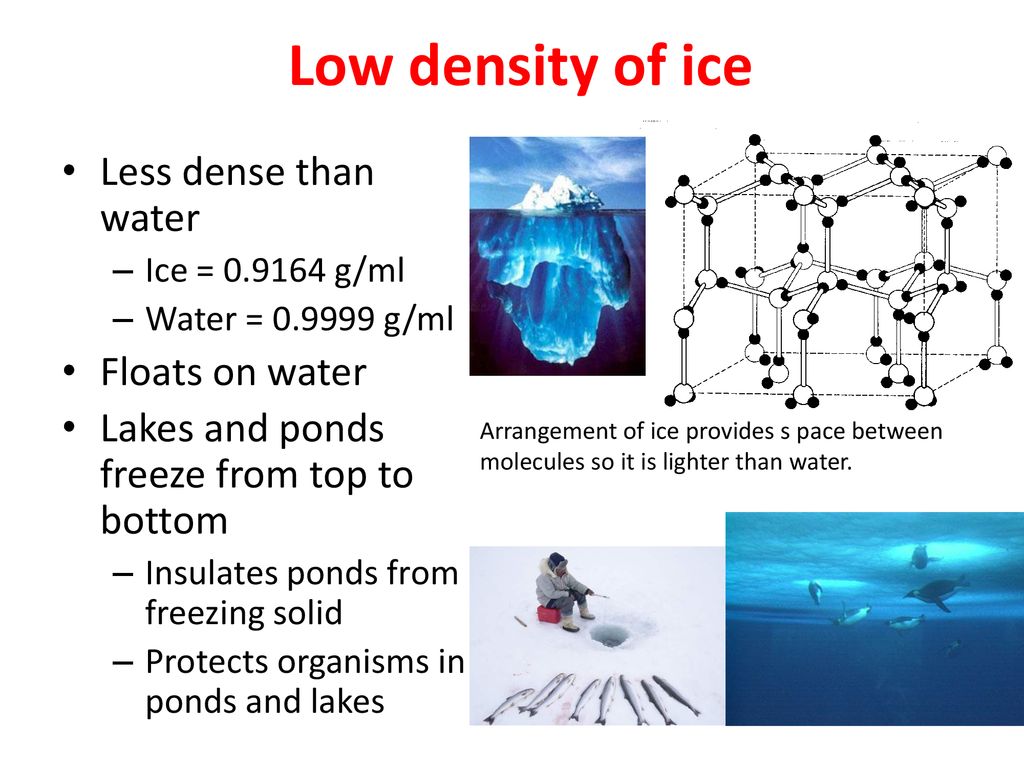 Source: slideplayer.com
Source: slideplayer.com
Solid water or ice is less dense than liquid water. When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. These bondings leave behind the larger amount of empty space due to which density decreases. As a result the molecules spread out and so ice can float in water. Water expands when it freezes making it less dense the lower the density the lighter it is.
 Source: quora.com
Source: quora.com
Something must be happening to. The question then becomes. This is mainly because of the hydrogen bonding present in the molecules of water. These bondings leave behind the larger amount of empty space due to which density decreases. Something must be happening to.
Source: quora.com
By staff writer last updated apr 2 2020 11 56 39 am et. Something must be happening to. By staff writer last updated apr 2 2020 11 56 39 am et. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. The simple answer is that ice is less dense than water.
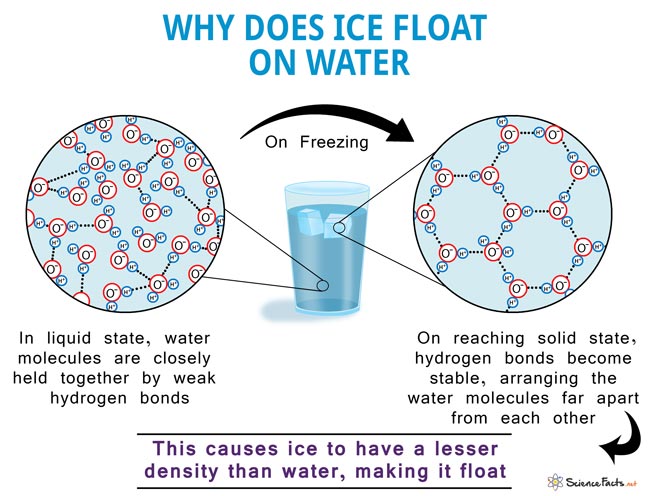 Source: sciencefacts.net
Source: sciencefacts.net
Ice is less dense than water. As water freezes it expands so it gets bigger but the mass in it still stays the same. When water freezes the molecules form a pattern because of the charge of each atom. The angle of the hydrogen oxygen bounds 105 degrees gives ice the special crystal structure. Something must be happening to.
 Source: thoughtco.com
Source: thoughtco.com
Solid water or ice is less dense than liquid water. The simple answer is that ice is less dense than water. The angle of the hydrogen oxygen bounds 105 degrees gives ice the special crystal structure. Water is unusual in that its maximum density occurs as a liquid rather than as a solid. As water freezes it expands so it gets bigger but the mass in it still stays the same.
 Source: reddit.com
Source: reddit.com
Ice is less dense than water. The question then becomes. As a result the molecules spread out and so ice can float in water. O and h. The angle of the hydrogen oxygen bounds 105 degrees gives ice the special crystal structure.
 Source: m.youtube.com
Source: m.youtube.com
When water freezes the molecules form a pattern because of the charge of each atom. The density of water is 1 gm cm 3. Water expands when it freezes making it less dense the lower the density the lighter it is. When water freezes the molecules form a pattern because of the charge of each atom. As water freezes it expands so it gets bigger but the mass in it still stays the same.
 Source: jagranjosh.com
Source: jagranjosh.com
Density is the mass per unit volume of a material. The simple answer is that ice is less dense than water. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. The density of water is 1 gm cm 3. As a result the molecules spread out and so ice can float in water.
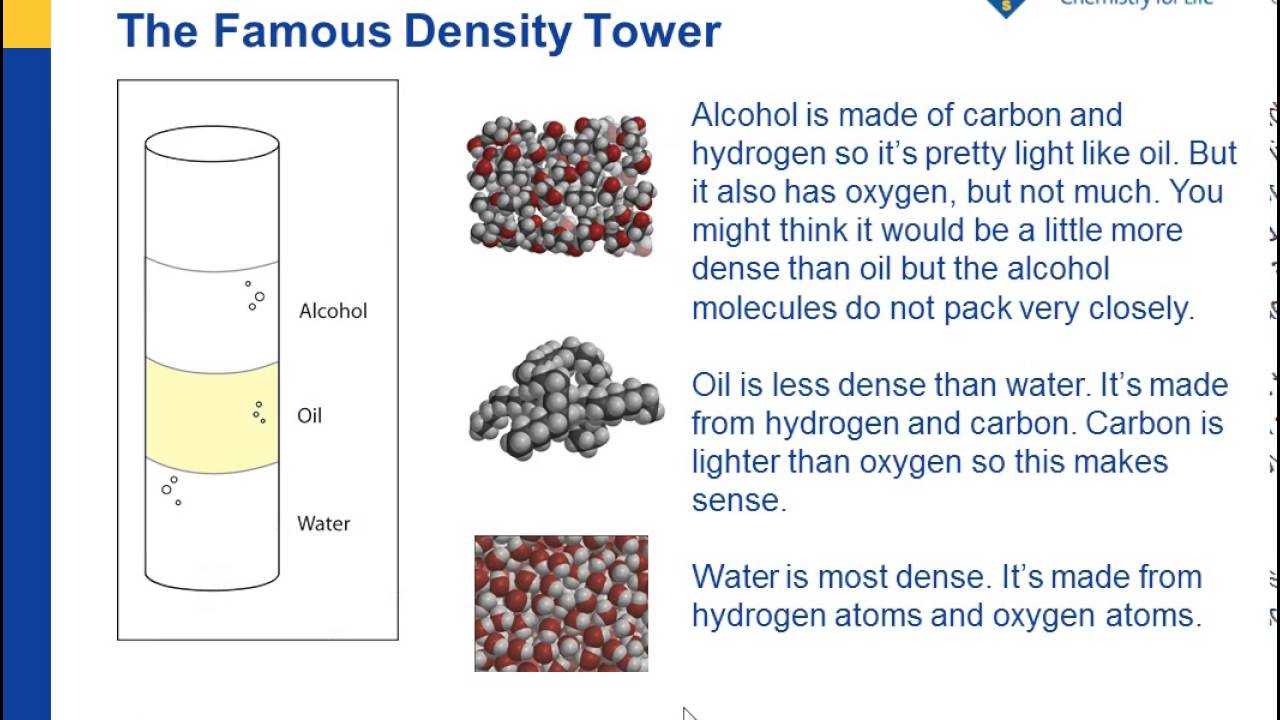 Source: youtube.com
Source: youtube.com
Water expands when it freezes making it less dense the lower the density the lighter it is. For all substances density changes with temperature the mass of material does not change but the volume or space that it occupies either increases or decreases with temperature. When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. Solid water or ice is less dense than liquid water. Something must be happening to.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Ice is less dense than water. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. Water is unusual in that its maximum density occurs as a liquid rather than as a solid. The additional space created reduces the density of the water as it freezes making ice less dense than water. The simple answer is that ice is less dense than water.
 Source: thoughtco.com
Source: thoughtco.com
When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. As a result the molecules spread out and so ice can float in water. The additional space created reduces the density of the water as it freezes making ice less dense than water. This means ice floats on water. The angle of the hydrogen oxygen bounds 105 degrees gives ice the special crystal structure.
 Source: usgs.gov
Source: usgs.gov
The question then becomes. As water freezes it expands so it gets bigger but the mass in it still stays the same. The additional space created reduces the density of the water as it freezes making ice less dense than water. The simple answer is that ice is less dense than water. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding.
Source: quora.com
When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. As water freezes it expands so it gets bigger but the mass in it still stays the same. As a result the molecules spread out and so ice can float in water. The ice is lighter than the water because the density of ice is lesser than that of water. This means ice floats on water.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why is ice lighter than water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.