Why is water such an important molecule to living things
Why Is Water Such An Important Molecule To Living Things. All these molecules are polar. Water is the colourless transparent fluid that is the basis of the fluids in the living organisms. Water is highly cohesive and adhesive. When water form hydrogen bonds with other substance the attraction is called adhesion.
 Physical Biochemistry From slideshare.net
Physical Biochemistry From slideshare.net
Metal salts dissolve in water. Chemical reactions of water. This is called cohesion. Because of hydrogen bonds water molecules develop strong intermolecular attraction between them. These water molecules attract more water molecules and hydrogen bonds form between them. To stay alive the organism takes in important materials for making energy while shuttling out toxic.
This is called cohesion.
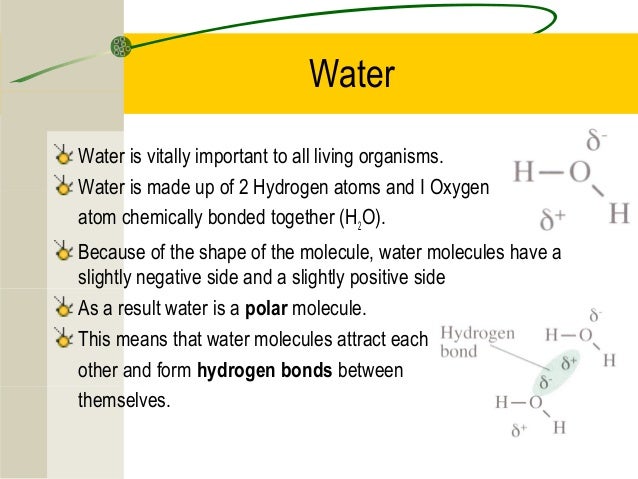
The chemical symbol of water is h2o. The chemical symbol of water is h2o. The oxygen atoms of water molecules are attracted to cations ions with a positive charge and water molecules surround it. Water also participates in building larger molecules in cells. Chemical reactions of water. These water molecules attract more water molecules and hydrogen bonds form between them.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
This means that each water molecule is made up of two hydrogen atoms bonded to a single atom of oxygen. At heart all life on earth uses a membrane that separates the organism from its environment. The specific arrangement of the hydrogen and oxygen atoms allow. Water is highly cohesive and adhesive. Water is directly involved in many chemical reactions to build and break down important components of the cell.
 Source: slideshare.net
Source: slideshare.net
The chemical symbol of water is h2o. Water is directly involved in many chemical reactions to build and break down important components of the cell. Metal salts dissolve in water. Photosynthesis the process in plants that creates sugars for all life forms requires water. Chemical reactions of water.
 Source: slideplayer.com
Source: slideplayer.com
The oxygen atoms of water molecules are attracted to cations ions with a positive charge and water molecules surround it. To stay alive the organism takes in important materials for making energy while shuttling out toxic. The oxygen atoms of water molecules are attracted to cations ions with a positive charge and water molecules surround it. This means that each water molecule is made up of two hydrogen atoms bonded to a single atom of oxygen. When water form hydrogen bonds with other substance the attraction is called adhesion.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
Water is highly cohesive and adhesive. These water molecules attract more water molecules and hydrogen bonds form between them. Water is important to living things because living things need water the most to be active another answerwater is a natural solvent. Most compounds with ionic bonding e g. Photosynthesis the process in plants that creates sugars for all life forms requires water.
 Source: slideplayer.com
Source: slideplayer.com
Water is important to living things because living things need water the most to be active another answerwater is a natural solvent. The specific arrangement of the hydrogen and oxygen atoms allow. Metal salts dissolve in water. The chemical symbol of water is h2o. Photosynthesis the process in plants that creates sugars for all life forms requires water.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
All these molecules are polar. Metal salts dissolve in water. At heart all life on earth uses a membrane that separates the organism from its environment. Chemical reactions of water. This is called cohesion.
 Source: expii.com
Source: expii.com
Most compounds with ionic bonding e g. When water form hydrogen bonds with other substance the attraction is called adhesion. The chemical symbol of water is h2o. Most compounds with ionic bonding e g. Water also participates in building larger molecules in cells.
 Source: slideplayer.com
Source: slideplayer.com
Here we explain what water is and give 20 compelling reasons why it is so important. Water is highly cohesive and adhesive. Photosynthesis the process in plants that creates sugars for all life forms requires water. Water is the colourless transparent fluid that is the basis of the fluids in the living organisms. The chemical symbol of water is h2o.
 Source: slideplayer.com
Source: slideplayer.com
Water is the colourless transparent fluid that is the basis of the fluids in the living organisms. This is called cohesion. The specific arrangement of the hydrogen and oxygen atoms allow. This means that each water molecule is made up of two hydrogen atoms bonded to a single atom of oxygen. The chemical symbol of water is h2o.

The oxygen atoms of water molecules are attracted to cations ions with a positive charge and water molecules surround it. Water is directly involved in many chemical reactions to build and break down important components of the cell. The specific arrangement of the hydrogen and oxygen atoms allow. Water is important to living things because living things need water the most to be active another answerwater is a natural solvent. To stay alive the organism takes in important materials for making energy while shuttling out toxic.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
Water is the colourless transparent fluid that is the basis of the fluids in the living organisms. The specific arrangement of the hydrogen and oxygen atoms allow. The chemical symbol of water is h2o. At heart all life on earth uses a membrane that separates the organism from its environment. These water molecules attract more water molecules and hydrogen bonds form between them.

Most compounds with ionic bonding e g. Photosynthesis the process in plants that creates sugars for all life forms requires water. Most compounds with ionic bonding e g. This means that each water molecule is made up of two hydrogen atoms bonded to a single atom of oxygen. Metal salts dissolve in water.
 Source: studylib.net
Source: studylib.net
The specific arrangement of the hydrogen and oxygen atoms allow. Metal salts dissolve in water. These water molecules attract more water molecules and hydrogen bonds form between them. This is called cohesion. Water is important to living things because living things need water the most to be active another answerwater is a natural solvent.
 Source: slideshare.net
Source: slideshare.net
Here we explain what water is and give 20 compelling reasons why it is so important. The chemical symbol of water is h2o. Water is the colourless transparent fluid that is the basis of the fluids in the living organisms. Most compounds with ionic bonding e g. This means that each water molecule is made up of two hydrogen atoms bonded to a single atom of oxygen.

Chemical reactions of water. Water also participates in building larger molecules in cells. To stay alive the organism takes in important materials for making energy while shuttling out toxic. Chemical reactions of water. Most compounds with ionic bonding e g.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why is water such an important molecule to living things by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.